Table of Contents
What is Chromatography?
Chromatography is a large family of analytical procedures used to separate, identify, and quantify components in a mixture. It is an effective technique in many scientific fields, including chemistry, biochemistry, pharmacology, environmental analysis, and forensic research.
The chromatographic concept is based on the differential distribution of components between two phases: stationary and mobile. The stationary phase can be either solid or liquid, whereas the mobile phase is usually either liquid or gas. The examined mixture is dissolved or dispersed in the mobile phase before being injected into the chromatographic apparatus.
The components of the mixture interact differently with the stationary phase when the mobile phase flows over or through it, depending on their physicochemical qualities such as polarity, size, charge, or affinity. Because of these interactions, the components separate at variable rates of migration or retention.
Because each component spends different amounts of time interacting with the stationary and mobile phases, the separation is achieved. Components having a higher affinity for the stationary phase interact more strongly and migrate more slowly, whereas components with a lower affinity for the stationary phase interact less and migrate faster.
To accomplish separation, several chromatographic procedures employ varied concepts. Among the most frequent forms of chromatography are:
- Gas Chromatography (GC): Gas chromatography (GC) separates volatile chemicals based on their partitioning between a stationary phase, which is commonly a high-boiling liquid coated on an inert solid support, and a gaseous mobile phase.
- High-Performance Liquid Chromatography (HPLC): This technique separates components by utilizing a liquid mobile phase and a stationary phase that can be a solid adsorbent or a liquid adsorbent mounted on a solid support.
- Thin Layer Chromatography (TLC): As previously said, it includes the separation of components utilizing a liquid mobile phase on a thin layer of adsorbent material coated on a plate.
- Ion Chromatography (IC): In the presence of an adequate mobile phase, this method separates ions based on their affinity for ion-exchange resins.
- Affinity Chromatography: This technique takes use of the particular interactions between a biological molecule, such as an enzyme or an antibody, and a ligand immobilized on the stationary phase to enable for selective separation and purification.
These are only a handful of the several chromatographic methods available. Each methodology has its own set of benefits, drawbacks, and applications, allowing scientists to select the best method for their individual analytical needs.
Chromatography is used to provide significant information on the composition, purity, and concentration of mixtures in various fields of research, quality control, and analysis. It is a key tool for comprehending complicated systems and has made substantial contributions to scientific developments in a variety of domains.
What is Thin Layer Chromatography?
Thin Layer Chromatography (TLC) is a technique for separating and identifying components in a mixture. It employs a finely split adsorbent solid or liquid dispersed on a plate, as well as a liquid mobile phase.
Non-volatile mixtures are extracted and evaluated using TLC. Typically, the experiment is performed on a sheet of aluminum foil, plastic, or glass that has been covered with a thin coating of an adsorbent substance. Aluminum oxide, cellulose, and silica gel are common adsorbents. Based on their chemical characteristics, these adsorbents can attract and retain various components of the mixture.
TLC separation occurs as the mobile phase, a liquid solvent or combination of solvents, travels up the plate by capillary action. The mobile phase transports the mixture’s components as it passes over the adsorbent layer. However, each component interacts with the adsorbent differently, resulting in varied degrees of retention.
When the separation is complete, the components of the mixture appear as discrete spots on the TLC plate. These locations are split vertically depending on the components’ differential retention. The retention factor (Rf) is a metric that quantifies the amount to which an adsorbent material retains a component.
The retention factor (Rf) is derived by dividing the sample spot’s distance traveled by the solvent front’s distance traveled:
(distance reached by sample spot) / (distance traveled by solvent front) = Rf
The Rf value is a unique attribute of each component that may be used to identify it. Different chemicals have varying affinities for the adsorbent and, as a result, varying Rf values. It is feasible to determine the components present by comparing the Rf values obtained from a sample to those of recognized chemicals.
The retention factor in TLC can be influenced by a number of things. These factors include the solvent system utilized, the amount of material spotted onto the plate, the kind of adsorbent employed, and the temperature at which the experiment is carried out. It is feasible to optimize the separation and obtain precise identification of the mixture’s components by carefully adjusting these factors.
Thin Layer Chromatography is commonly used in medicines, forensics, food chemistry, and environmental investigation. It is popular because to its speed, low cost, simplicity, and ease of use. These benefits make TLC an appealing technique for regular and qualitative examination of mixtures in a variety of laboratory settings.
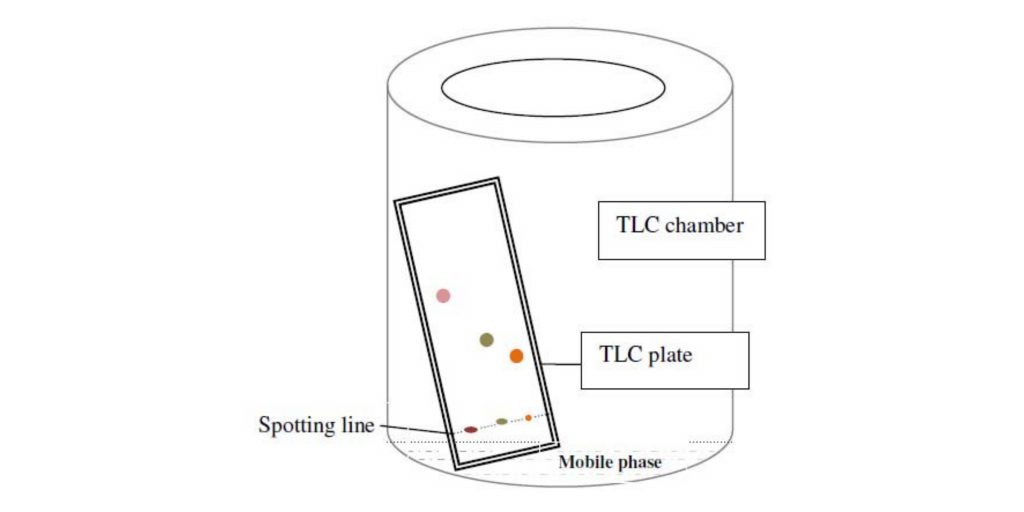
Thin Layer Chromatography Principle – Principle of thin layer chromatography
- The Thin Layer Chromatography (TLC) concept is based on the relative affinity of substances for the stationary and mobile phases. TLC is done on a glass, plastic, or aluminum foil plate that has been covered with a thin layer of adsorbent material such as silica gel, aluminum oxide (alumina), or cellulose. This adsorbent layer is known as the stationary phase.
- When a sample is put to a TLC plate, capillary action draws a solvent or a combination of solvents, known as the mobile phase, up the plate. As the mobile phase flows across the surface of the stationary phase, the individual chemicals in the sample interact with it differently depending on their affinity.
- TLC separation principles can be adsorption chromatography, partition chromatography, or a combination of both, depending on the adsorbent utilized and the type of the solvents used. Because they spend more time interacting with the adsorbent material, components having a higher affinity for the stationary phase will move more slowly. Components having a lower affinity for the stationary phase, on the other hand, will travel quicker due to weaker interactions.
- As the separation process progresses, various components of the mixture separate and appear as discrete spots on the TLC plate at varying levels of travel. The isolated compounds are represented by these dots. Appropriate detection techniques are used to determine the type or character of these components. Among the most common detection methods are ultraviolet (UV) viewing, the use of chemical reagents, and the use of fluorescent dyes.
- The components can be identified by comparing the migration distances or retention factors (Rf values) of the spots collected from the sample to those of recognized chemicals. Each compound has its own Rf value, which is the ratio of the compound spot’s distance traveled to the solvent front’s distance traveled.
- To summarize, the TLC concept is based on the differential migration of molecules on the TLC plate depending on their affinity for the stationary and mobile phases. Components having higher interactions with the stationary phase move more slowly, whereas components with weaker connections move quicker. Using proper detection techniques, the separated components are viewed and recognized.
Components of Thin Layer Chromatography (TLC) – Instrumentation of Thin Layer Chromatography
Thin Layer Chromatography (TLC) entails numerous critical components that are required for efficient chemical separation and analysis. These elements are as follows:
- TLC Plates: These plates are pre-fabricated and feature a chemically inert surface. Glass, plastic, or aluminum foil plates are commonly covered with a thin coating of stationary phase material. The stationary phase is administered consistently in fine particle size, resulting in consistent separation.
- TLC Chamber: The TLC chamber is where the TLC plate is developed. It provides an enclosed habitat in which consistent conditions for appropriate spot growth are maintained. The chamber prevents solvent evaporation and maintains the procedure free of dust and external contaminants.
- Mobile Phase: The mobile phase is made up of a solvent or a combination of solvents. It is in charge of transporting the sample and aiding component mobility on the TLC plate. To guarantee optimum separation, the mobile phase should be particulate-free and of the greatest purity. It is critical to use solvents that are chemically inert to the sample and stationary phase.
- Filter Paper: Moistened with the mobile phase, filter paper is inserted inside the TLC chamber. The filter paper’s aim is to provide a consistent rise of the mobile phase along the length of the stationary phase. This guarantees that compound separation proceeds equally and consistently.
These components collaborate to allow for successful separation and analysis in TLC. The TLC plates offer a stable surface with a thin layer of stationary phase, while the TLC chamber provides a suitable environment for growth. The mobile phase transports the sample, allowing the chemicals to move over the TLC plate. The inclusion of filter paper contributes to the uniform migration of the mobile phase.
Scientists may produce consistent and precise separations in TLC by carefully choosing and optimizing these components, allowing the identification and investigation of diverse chemicals in a mixture.
Procedure of Thin Layer Chromatography (TLC)
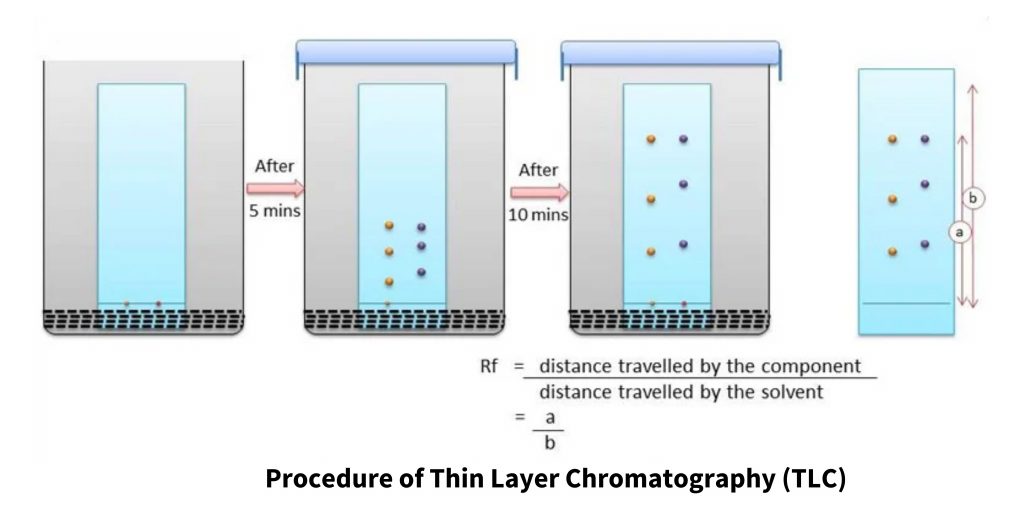
The procedure of Thin Layer Chromatography (TLC) involves several steps to achieve effective separation and visualization of the components. The general procedure is as follows:
- Preparation of the TLC Plate: The stationary phase is applied uniformly onto the plate surface. Nowadays, ready-made TLC plates with a pre-coated stationary phase are commonly used, eliminating the need for manual application and drying.
- Marking the Plate: Using a pencil, a thin line is drawn at the bottom of the TLC plate. This line serves as a reference for applying the sample spots.
- Application of Sample: The sample solutions are carefully applied onto the spots marked on the line at equal distances. This is typically done using a micro-capillary pipette or a micropipette to ensure precise application of the samples.
- Preparation of TLC Chamber: The mobile phase, which consists of the appropriate solvent or solvent mixture, is poured into the TLC chamber. The level of the mobile phase should be a few centimeters above the bottom of the chamber.
- Adding a Moistened Filter Paper: A filter paper saturated with the mobile phase is placed on the inner wall of the TLC chamber. This helps maintain equal humidity within the chamber and prevents edge effects during the development process.
- Placement of the TLC Plate: The TLC plate prepared with the sample spots is placed inside the chamber with the side containing the sample line facing the mobile phase. The chamber is then closed with a lid to ensure a controlled environment.
- Development of the Plate: The TLC plate is allowed to develop in the chamber. The plate is immersed in such a way that the sample spots are well above the level of the mobile phase but not directly immersed in the solvent. Sufficient time is given for the compounds to separate and migrate on the plate.
- Removal and Drying: Once the desired separation is achieved, the TLC plate is carefully removed from the chamber. It is then allowed to dry completely before further analysis.
- Visualization of Spots: The developed TLC plate is visualized to observe the separated spots. Various techniques can be employed for visualization, depending on the nature of the sample. Common techniques include:
- UV Light: The TLC plate can be examined under UV light, and the separated spots may appear as fluorescent spots due to natural fluorescence or the use of fluorescent dyes in the sample.
- Iodine Staining: Iodine vapor can be used to detect carbohydrates, as it turns black upon contact with carbohydrates on the TLC plate.
- KMnO4 Stain: Organic molecules can be visualized using potassium permanganate (KMnO4) stain.
- Ninhydrin Reagent: Amino acids and proteins can be detected using ninhydrin reagent, which causes the spots to turn purple or blue.
By following this procedure and utilizing appropriate visualization techniques, TLC allows for the separation and identification of components in a mixture, providing valuable information about their presence and relative quantities.
What is Retention Factor (Rf ) Value
The retention factor (Rf) value in Thin Layer Chromatography (TLC) is a quantitative metric used to explain the behavior of a molecule throughout the separation process. Each molecule has a unique value under certain chromatographic circumstances.
The ratio of the distance traveled by the compound spot to the distance traveled by the solvent front determines a compound’s Rf value. The following equation may be used to compute it:
Rf = compound spot distance traveled / solvent front distance traveled
The Rf value is a dimensionless quantity ranging from 0 to 1. A compound with an Rf value close to zero has a higher affinity for the stationary phase and so travels less with the mobile phase. A compound with an Rf value near to one, on the other hand, indicates that it has a lesser affinity for the stationary phase and hence travels more with the mobile phase.
It is vital to remember that the Rf value is affected by a number of variables that must be maintained constant in order to compare multiple trials accurately. The solvent system (composition and polarity), the adsorbent material (such as silica gel or alumina), the thickness of the adsorbent layer, the amount of material spotted, and the temperature are all aspects to consider. Maintaining constant circumstances between experiments allows Rf values to be readily compared.
However, due to the difficulties in precisely regulating all of these variables, relative Rf values are frequently considered. The Rf values of the compounds of interest are presented relative to the Rf value of a suitable reference compound. This enables more accurate comparisons of different TLC plates and investigations.
The Rf value is an important parameter in TLC analysis because it may be used to identify and classify compounds by comparing their Rf values to those of recognized substances. The Rf value may be used to understand and analyze TLC findings by utilizing proper standards and consistent chromatographic settings.
Applications of Thin Layer Chromatography (TLC)
Thin Layer Chromatography (TLC) has several uses in a variety of industries. The following are some of the most important TLC applications:
- Monitoring Reaction Progress: TLC may be used to monitor reaction progress by monitoring the disappearance of starting materials and the emergence of new compounds during the reaction.
- Identification of chemicals: TLC is often used to identify the chemicals contained in a mixture. The components can be detected by comparing the migration of sample spots to established standards and comparing their retention factors (Rf values).
- Determining Substance Purity: TLC may be used to determine the purity of a material by comparing the chromatographic profile of the sample to that of a pure reference substance.
- Ceramides and Fatty Acid Analysis: TLC is used for evaluating and isolating ceramides and fatty acids in a variety of samples, including biological materials.
- Detection of Pesticides and Insecticides: TLC is used to detect and analyze pesticides and insecticides in food and water samples.
- Fiber Dye Composition Analysis in Forensics: TLC is important in the forensic study of fibers to identify the dye composition, which aids in the identification and comparison of fibers in criminal investigations.
- Radiochemical Purity Assay: TLC is used to evaluate the radiochemical purity of radiopharmaceuticals, assuring the quality and safety of these substances utilized in medical imaging and therapy.
- Medicinal Plant Identification: TLC is used in the identification of medicinal plants and their contents, which aids in the quality control and verification of herbal remedies.
- Qualitative Testing of Medicines: TLC is used for qualitative testing of different drugs, including sedatives, local anesthetics, anticonvulsants, tranquilizers, analgesics, antihistamines, steroids, and hypnotics.
- Biochemical Analysis: TLC is useful in biochemical analysis for separating and isolating metabolites from biological fluids such as blood plasma, urine, serum, and other bodily fluids.
- Natural Product Identification: TLC is used for the identification of natural products such as essential oils, volatile oils, fixed oils, glycosides, waxes, alkaloids, and other bioactive substances.
- Separation of Multicomponent Pharmaceutical Formulations: TLC is commonly utilized in the pharmaceutical sector for the separation and analysis of complicated multicomponent medicinal compositions.
- Purification of Samples: TLC may be used to purify samples by comparing the sample to a genuine reference sample and choosing the required fractions based on their migration.
- Food and Cosmetic Industry: TLC is used in the food and cosmetic industries to separate and identify colors, sweetening agents, preservatives, and other components used in the manufacturing of food and cosmetic goods.
- Reaction Completeness: TLC may be used to assess if a chemical reaction has completed by comparing the starting ingredients and products on the TLC plate.
TLC is an extremely flexible and frequently used method, with applications ranging from chemical analysis and quality control to forensic investigations and natural product identification. Its ease of use, low cost, and wide range of applications make it a valuable instrument in a variety of scientific areas.
Advantages of Thin Layer Chromatography (TLC)
Thin Layer Chromatography (TLC) has various features that contribute to its popularity and extensive use. Here are some important benefits of TLC:
- Simplicity and Short Development Time: TLC is a reasonably simple technology that does not require complicated apparatus and has a short development time. The procedure includes applying the sample, developing the plate, and seeing the separated chemicals. Its development period is minimal, allowing for speedy analysis and outcomes.
- Simple Visualization of Separated Compound Spots: TLC plates make it simple to see separated compound spots. The different substances display as separate dots, making it easy to visually assess and comprehend the data. This visual identification assists in the fast assessment of a mixture’s composition.
- Effective Compound Isolation: TLC is useful for isolating the majority of chemicals present in a mixture. Once separated on the TLC plate, the chemicals can be readily scraped or eluted off the plate for further investigation or purification. This allows researchers to get pure molecules for further investigation.
- Faster Separation and High Selectivity: TLC provides a speedier separation procedure and high selectivity as compared to other chromatographic methods. It can separate compounds efficiently based on even minor changes in their chemical characteristics, resulting in obvious separation and distinct spots. TLC has a high selectivity, enabling for successful separation and analysis of complicated mixtures.
- Simple Purity Standards Assessment: TLC is a simple method for determining the purity of a sample. The purity of the sample may be easily determined by comparing the separated chemicals on the TLC plate to recognized standards. This is very valuable in quality assurance and control operations.
- Cost-Effectiveness: TLC is a very inexpensive chromatographic method. TLC equipment is generally inexpensive, and consumables like as TLC plates and solvent solutions are widely accessible and reasonably priced. As a result, TLC is a viable option for regular analysis and large-scale applications.
To summarize, the benefits of Thin Layer Chromatography (TLC) are its simplicity, quick development time, simple visualization of separated compounds, efficient chemical isolation, speedier separation process, high selectivity, ease of assessing purity standards, and cost-effectiveness. TLC is a flexible and frequently utilized technique in a variety of scientific domains, including medicines, forensics, food analysis, and natural product research, due to these characteristics.
Limitations of Thin Layer Chromatography (TLC)
There are certain limits to Thin Layer Chromatography (TLC) that should be noted while using this technique. These constraints are as follows:
- Inability to Differentiate Enantiomers and Some Isomers: TLC can’t tell the difference between enantiomers (mirror-image isomers) and some structural isomers. It lacks the chiral discrimination required to efficiently separate enantiomers, making it less appropriate for enantiomeric analysis.
- Dependence on Known Rf Values: Before using TLC to identify specific compounds, the Rf values of the compounds of interest must be known. For proper identification, previous information or reference standards are required, restricting the use of TLC for unknown chemicals.
- Limited Separation Length: TLC plates have a comparatively brief stationary phase as compared to other chromatographic processes. As a result, the length of separation achieved using TLC is restricted, which may result in overlapping or poorly resolved spots, particularly in complicated combinations.
- Difficulty in Result Reproducibility: The reproducibility of TLC findings might be difficult. Variations in chromatograms can be caused by variances in plate preparation, sample application, development conditions, and detection procedures. To get consistent and repeatable findings, experimental conditions must be carefully controlled and standardized.
- Environmental Sensitivity: Because TLC is an open system, it is susceptible to environmental conditions such as humidity and temperature. Changes in these parameters can impact compound migration and separation on the TLC plate, influencing the results’ reliability and repeatability.
- Higher Detection Limit: When compared to other chromatographic methods, TLC offers a higher detection limit. As a result, it may not be suited for identifying chemicals in trace levels. If lower detection limits are necessary, more sensitive chromatographic procedures may be utilized.
- Qualitative Analysis Only: TLC is essentially a qualitative analytical method that provides information about the presence or absence of certain chemicals in a mixture. Because the spots on the TLC plate do not exactly correspond with chemical concentrations, it is not suited for accurate quantitative measurement.
Despite these limitations, TLC is a powerful method for fast qualitative analysis, compound screening, and compound separation in a variety of disciplines. Other chromatographic procedures with stronger resolving power and sensitivity may be utilized for more sophisticated separation and analytical requirements.
FAQ
What is Thin Layer Chromatography (TLC)?
TLC is a chromatographic technique used for the separation and identification of compounds in a mixture based on their relative affinities to the stationary and mobile phases.
What are the components required for a TLC experiment?
The essential components include TLC plates (with a stationary phase), TLC chamber, mobile phase, sample solutions, and suitable detection techniques.
How does TLC work?
TLC relies on the differential migration of compounds between the stationary phase (adsorbent material) and the mobile phase (solvent or solvent mixture) to achieve separation based on their affinities.
What is the role of the stationary phase in TLC?
The stationary phase provides a surface for the adsorption or partition of the compounds being separated, allowing for differential movement based on their affinities.
What are the commonly used stationary phases in TLC?
Silica gel, alumina, and cellulose are frequently used as stationary phases in TLC.
What is the Rf value in TLC?
The retention factor (Rf) is a measure of the relative migration distance of a compound compared to the solvent front. It is calculated as the ratio of the distance traveled by the compound to the distance traveled by the solvent front.
How is TLC used for compound identification?
TLC can be used for the comparison of Rf values obtained from unknown compounds with those of known reference compounds to aid in identification.
What are the advantages of TLC?
Some advantages include its simplicity, short development time, easy visualization of spots, ability to isolate compounds, faster separation, higher selectivity, assessment of purity, and cost-effectiveness.
What are the limitations of TLC?
Limitations include difficulties in differentiating enantiomers and some isomers, reliance on pre-known Rf values for compound identification, limited separation length compared to other techniques, variability in reproducibility, and challenges with humidity and temperature control.
What are the applications of TLC?
TLC finds applications in various fields, such as monitoring reactions, identifying compounds, assessing substance purity, analyzing ceramides and fatty acids, detecting pesticides in food and water, studying medicinal plants, and analyzing multicomponent pharmaceutical formulations.
References
- Snyder, L. R., Kirkland, J. J., & Dolan, J. W. (2011). Introduction to modern liquid chromatography. John Wiley & Sons.
- Braithwaite, A., & Smith, F. (2004). Chromatographic methods. Springer Science & Business Media.
- Stahl, E. (1969). Thin-layer chromatography: A laboratory handbook. Springer Science & Business Media.
- Sherma, J., & Fried, B. (2003). Handbook of Thin-Layer Chromatography. Marcel Dekker.
- Ettre, L. S. (Ed.). (1996). History of analytical chemistry. Elsevier Science.
