Table of Contents
Milk and milk products that provide nutrition have been of fundamental importance since ancient times. These are aids for improving the economic status of farmers and sellers, as well as the health of consumers. However, microbial contamination may cause these products to spoil, resulting in the potential loss of not only producers and sellers, but also consumers. As a result, there is an urgent need to investigate the causes of spoilage in addition to their preservation for long-term use.
Due to its highly perishable and putrid qualities, as well as the presence of undesirable pathogens in it, milk is subjected to a variety of preservation procedures. Pasteurization, commercial sterilization, fermentation, dehydration, refrigeration, and freezing are all components of the dairy processing procedure. Marketed milk products include heated milk, yogurt, kefir, kumiss, cream, butter, cheese, condensed and dried milk, and others. When milk and milk products spoil, off-flavors and odours develop, as well as changes in texture and appearance.
Contamination of Milk and Milk Products
On the Farm
- Milk contains relatively few bacteria when it exits the udder of a healthy cow, and these bacteria often do not multiply under normal handling conditions.
- Nonetheless, micrococci and streptococci have been isolated from aseptically withdrawn milk. During normal milking, milk is susceptible to contamination from the animal, particularly the outside of the udder and surrounding areas.
- From this source, bacteria found in manure, soil, and water may enter. Before milking, this contamination is decreased by clipping the cow, particularly the flanks and udder, grooming the cow, and rinsing the udder with water or a germicidal solution.
- The contamination of cows with soil, water, and dung is reduced by paving and draining barnyards, preventing cows from standing in pools of stagnant water, and removing manure from barns and milking rooms.
- Dairy utensils and milkcontact surfaces, including the milk pail or milking machines, where applicable, strainers, milk cans or pipelines, and the bulk-milk chiller, are likely the two most major sources of contamination.
- If dairy utensils or milk-contact surfaces are not adequately cleaned, sterilised, and dried, bacteria can grow in huge numbers in the milklike residue and enter the next milk to come into contact with these surfaces.
- From these sources, undesirable bacteria include lactic streptococci, coliform bacteria, psychrotrophic gram-negative rods, and thermodurics, such as micrococci*, enterococci*, bacilli, and brevibacteria*.
- In general, these bacteria thrive in milk and hence threaten its shelf-life. When adequately cleaned and sanitised, utensils and milk-contact surfaces add a small number of germs per millilitre of milk. However, in extremely unsanitary conditions, these sources can raise the milk’s bacterial count by millions per millilitre.
- As sanitising agents, quaternary ammonium compounds tend to increase the proportion of gram-negative rods (psychrotrophs, coliforms) on utensils, whereas hypochlorites promote gram-positive bacteria (micrococci*, bacilli).
- Modern dairy utensils and milk-contact surfaces, including milking machines, pipelines, and bulk-milk refrigerators, are intended to facilitate cleaning, disinfecting, and drying.
- Farm bulk-milk coolers are also outfitted with superior refrigeration capacity and agitation to guarantee that the milk is cooled properly.
- The hands and arms of the milker or dairy staff, the air in the barn or milking room, and insects are additional potential sources of contamination.
- Typically, these sources contribute a negligible amount of bacteria, although they may be a source of pathogens or spoiling germs.
- The milk quality will be affected by the quality of the water used in the milking parlour for washing, rinsing, etc.
- The quantity of bacteria per millilitre of milk added from diverse sources is contingent upon the precautions taken to prevent contamination.
- For instance, if care are followed and a milking equipment is utilised, the exterior of the cow contributes relatively few organisms to the milk. However, under really poor conditions, thousands per millilitre could enter the milk.

In Transit and at the Manufacturing Level
- Other sources of contamination after the milk has left the farm include the tanker truck, transfer pipes, sampling instruments, and equipment at the market-milk plant, cheese factory, condensery, and other processing facilities.
- Again, the milk contact surfaces are the most major contamination sources. Possible sources of bacteria include pipelines, vats, tanks, pumps, valves, separators, clarifiers, homogenizers, coolers, strainers, stirrers, and fillers.
- The amount or level of contamination from each of these sources is contingent upon the cleaning and sanitising techniques used. Additionally, staff, especially their hands and arms, may be a source of contamination and infections.
- The paper used to package fluid milk is a significant source of contamination as well.
- It is important to remember that the number and types of organisms in milk and other dairy products can be increased by contamination or by the expansion of existing organisms.
- Both are prevented through production, handling, storage, and manufacturing procedures.
Preservation of Milk and Milk Products
- Milk has such a delicate flavour and is so easily altered that many preservation methods cannot be utilised without generating an undesired change or, at best, producing a new food product.
- In actuality, the majority of milk and cream-based products evolved to improve their shelf-life.
- Thus, nomadic cultures discovered that milk that had been fermented with lactic acid could be stored for extended periods, but we currently create fermented milks for their distinctive body and flavour.
- It was discovered that milk curds may be treated in a variety of ways to extend their shelf life; hence, we today manufacture a variety of cheeses with distinct properties.
- Milk and milk products, which serve to illustrate the majority of food preservation and spoiling concepts, have been the subject of more research than most other foods, and countless manuals and reference books have been written on the topic.
- These references, some of which are given at the end of this chapter, should be consulted for additional information that is beyond the scope of this article.
1. Asepsis
- It is crucial for the preservation of milk to prevent contamination to the greatest extent possible.
- When fewer microorganisms are present, especially those that proliferate quickly in milk, preservation quality is typically enhanced.
- Although the type of microorganisms present is of utmost importance, in general, the smaller the initial overall microbial load, the longer milk will keep.
- For instance, a low microbial burden, particularly the number of spores present, is an essential factor in milk to be sterilised by ultrahigh-temperature or commercial procedures.
- Since the quantity of bacteria in milk is indicative of the sanitary precautions and careful handling applied during manufacturing, the bacterial content of milk is used to measure its sanitary quality, and milk has historically been graded based on some technique of determining the number of bacteria.
- It has been stressed that the outside of the cow and milk-contact surfaces are potential sources of both high numbers and undesirable types of bacteria.
- Particularly unwanted in market milk are bacteria that thrive in milk, such as lactics and coliforms; psychrotrophs, which thrive at the refrigerated temperatures at which milk is often stored; thermodurics, which survive the pasteurisation process; and, of course, human pathogens.
- When milk is used as the substrate for microbial fermentation, as in the production of fermented milks or cheese, it is crucial that microorganisms capable of competing with the starter germs and causing product faults are present.
- Consequently, coliform bacteria, anaerobes, and yeast can induce flatulence and off-flavors in these goods.
- Other organisms may hinder the desirable starting culture or create body, texture, or flavour faults.
- High-heat-treatment milks, such as evaporated milk and sweetened condensed milk, may be susceptible to spoiling if they include heat-resistant microbes or spores.
- Packaging keeps germs away from bottled milk, fermented milks, packed butter, canned milk, dry milk, and packaged cheese, as do plastic, wax, or other protective coatings on completed cheeses.
- The bacterial quality of the paper used to make paper milk cartons has been evaluated.
- Typically, packing material contributes a negligible amount to the total microbial load of the final product.
2. Removal of Microorganisms
- Once germs have invaded milk, it is difficult to efficiently eliminate them. Some germs will be removed from milk using centrifugation, as in clarification or separation.
- At 10,000 g, high-speed centrifugation removes around 99 percent of the spores and more than half of the vegetative cells and protein of bacteria.
- Bactofugation, the centrifugal process used to remove germs from milk, is not widely utilised in the commercial sector.
- Molds can be physically removed from the surface of certain types of cheese during the ageing process by scraping or occasional washing, but physical removal is otherwise difficult.
3. Use of Heat
a. Pasteurization and Ultra Pasteurization
- Because milk and cream are so easily altered by heat, they are preserved by a process known as pasteurisation.
- Historically, milk was initially treated with heat to extend its shelf life. As it became apparent that milk may be a cause of food-borne illness, pasteurisation became an essential precaution.
- Pasteurization of commercial milk aims to (1) eliminate all microorganisms that may enter the milk and be passed to humans, and (2) improve the milk’s shelf-life.
- This heat treatment should ideally not have a negative impact on the flavour, appearance, nutritional characteristics, or creaminess of the milk.
- When milk is pasteurised for the production of cheese or when cream is pasteurised for the production of butter, a third objective is the destruction of microorganisms that would interfere with the activities of desirable organisms, such as the starter culture, or cause product deficiency or spoilage.
- The cheesemaker is also anxious that the heating process would not compromise the milk’s curdling qualities.
- Additionally, the heat treatment of cream eliminates lipases that may cause butter to degrade during storage.
- The first widespread method of pasteurising milk entailed heating the milk to 60 degrees Celsius in big tanks or vats for at least 20 minutes.
- Coxiella burnetii, a rickettsia responsible for Q fever that may be transferred in milk, was eradicated by lowering the holding temperature to 61.7 °C for 30 minutes and then to 62.8 °C for 30 minutes.
- This was referred known as vat pasteurisation and was not a continuous process. Using plate heat exchangers and a continuous operation entails the high-temperature-short-time (HTST) pasteurisation process at a minimum temperature of 72 degrees Celsius for at least 15 seconds.
- The HTST system is currently the most popular commercial pasteurisation method. The majority of processors, however, heat their products beyond the minimum temperature and time requirements.
- For instance, many pasteurise milk at temperatures nearing 79 degrees Celsius for up to twenty-five seconds. The primary purpose is to reduce total microbial load and consequently increase the product’s shelf life.
- Recent disease outbreaks linked to milk and other dairy products have suggested Listeria monocytogenes as the agent responsible. It was unclear if the minimal periods and temperatures of HTST pasteurisation were sufficient to eradicate L. monocytogenes from milk and other dairy products.
- Very high temperature (VHT) and ultrahigh-temperature (UHT) systems are terms used to describe milk and milk product heat-treatment technologies that surpass pasteurisation.
- There appears to be no exact definition for a VHT system. According to the International Dairy Federation, UHT methods typically involve pasteurisation techniques with temperatures of at least 130 degrees Celsius and holding periods of at least one second. States for fluid milk.
- To be labelled “ultrapasteurized,” a product must have been cooked to 137.8 degrees Celsius or more and held there for at least two seconds, according to current federal identification regulations. UHT systems are used to process cream for whipping, coffee cream, and half-and-half.
- The present disadvantage of UHT systems is that the intense heating required may alter the nutritional and organoleptic qualities of the product.
- Direct-heating technologies, including a stem-injection-into-milk process and a milk-injected-into-steam process, known as a steam-injection technique and a steam-infusion technique, are the most prevalent UHT systems.
- Following the heat treatment in a sterile vacuum chamber, the additional steam or excess water is extracted in both of these systems.
- When this form of heat treatment is combined with aseptic packaging, the resulting category of products is commonly known as “sterilised milk” or “sterilised cream.” This classification does not imply absolute sterility, but rather a commercially sterile product.
- These items have an exceptionally lengthy shelf life. The sterilised fluid milk or milk product must be packaged in containers that preserve the “sterility” and shelf life of the product.
- Some American processors guarantee that their sterilised milk products have a shelf life longer than six weeks. With the correct combination of effective sterilisation, aseptic packing, and adequate refrigeration, it is not uncommon for these types of items to have a shelf life of more than 90 days.
- The percentage of reduction in the number of microorganisms in milk following pasteurisation relies on (1) the pasteurisation temperature, (2) the holding duration, (3) the total number of bacteria, and (4) the fraction of the total microbial load that consists of sporeformers or thermoduric organisms.
- Generally speaking, the traditional HTST technology may reduce the amount of germs in milk by 90 to 99 percent. After pasteurisation, milk is swiftly cooled to 7.2 degrees Celsius or below.
- The shelf life of pasteurised milk is dependent on the storage temperature and the amount and types of bacteria that survive the pasteurisation process.
- During pasteurisation, more heat is applied to butter-making cream than to market cream. The holding approach entails heating at 71.1 C or more for 30 minutes, whereas the HTST method requires heating at 87.7 to 93.3 C for a few seconds.
- Cream is more protective against organisms than milk, and butter-making cream is more likely to contain microorganisms than most milk lots.
- Vacreation is a method for rapidly heating cream that involves injecting steam or a combination of steam injection and evacuation.
- In essence, the forewarming at 71 to 100 C for 10 to 30 minutes and evaporation processes at 48.9 to 57.2 C in the production of sweetened condensed milk are pasteurisation processes that kill all pathogens and should remove all organisms that could contaminate the final canned product.
- Condensed milk in bulk is subjected to what amounts to a high-temperature pasteurisation. Preheating the milk at temperatures between 65.6 and 76.7 degrees Celsius prior to evaporation kills numerous organisms, and superheating the condensed result, which is more concentrated than evaporated milk, at temperatures between 82.2 and 93.3 degrees Celsius kills even more.
- This item must be immediately cooled, stored at a chilling temperature, and utilised quickly. Preheating milk prior to drying, as described in the following section, is in effect pasteurisation.
- The melting and blending of cheese at 65.6 C or above during the production of processed cheese constitutes a pasteurisation procedure that eliminates the majority of bacteria contained in the original cheeses.
- As emulsifiers, phosphates, citrates, and tartrates are added. In milk, conventional pasteurisation should eliminate all yeasts and moulds and the majority of bacterial vegetative cells.
- The surviving bacteria, known as thermodurics, belong to a variety of distinct bacterial groups, of which only the most significant will be addressed. (1) the high-temperature lactics, e.g., the enterococci; Streptococcus thermophilus, high-temperature lactobacilli, such as Lactobacillus bulgaricus* and L. lactis*; and species of Microbacterium; and (2) certain species of Micrococcus* are the most important non-spore-forming bacteria.
- Some Streptococcus and Lactobacillus species are both thermophilic and thermoduric.
- The spore-forming thermodurics fall into two main groups: (1) species of Bacillus, i.e. aerobic to facultative spore-forming bacilli, of which B. cereus (Proteolytic) is typically the most prevalent, but B. licheniformis, B. subtilis (proteolytic), B. coagulans (thermophilic), B. polymyxa (gas-forming), and other species are occasionally significant; and (2) species of Clo The majority of those growing in milk produce gas.
- Other bacteria may survive pasteurisation, but they do not thrive in milk. As processors continue to boost pasteurisation temperatures to extend shelf life, the significance of spore-forming bacteria grows.
b. Steam under Pressure
- Milk that has been evaporated is sealed in jars and then heated by steam under pressure, frequently with rolling or agitation.
- Prior to evaporation, a milk temperature of 93 to 100 degrees Celsius or above kills all but the most resistant bacterial spores.
- The processing of sealed cans of evaporated milk at temperatures between 115 and 118 degrees Celsius for 14 to 18 minutes resulted in a commercially sterile product.
- Some heat-cool-fill techniques, such as the Martin method, have been applied to the processing of whole milk cream and whole milk concentrate in cans.
4. Use of Low Temperatures
With the exception of canned milk and dry milk, low temperatures are required to preserve the majority of dairy products; in many cases, this is the most significant component.
a. Refrigerated Storage
- It is crucial for the production of high-quality milk that the milk is chilled quickly after being extracted from the cow.
- The Grade A Pasteurized Milk Ordinance of the United States Public Health Service mandates that Grade A raw milk intended for pasteurisation must be cooled to 10 C or below within two hours of being drawn and maintained at that temperature until processed.
- Newly pasteurised milk must be chilled to 7.2 degrees Celsius or less and maintained at that temperature. Ideally, it should be cooled to temperatures far below 7.2 degrees Celsius.
- For instance, bulk-milk coolers on farms can rapidly cool milk to 3.0 to 4.5 C or lower and maintain that temperature, with the exception of brief intervals when fresh milk is introduced, during which the temperature does not typically exceed 7.2 C.
- In addition, it is not uncommon for the temperature of milk as it is being poured into milk cartons or bottles to approach 2 to 3 degrees Celsius.
- During storage on the farm, in the truck or tank during transport to the processing plant or receiving facility, and during storage there, milk is kept at refrigeration temperatures.
- Until consumption, milk or related products should be refrigerated during storage in the plant or on the retail market, during delivery, and in the home or restaurant.
- After production, fermented milks and unripened cheeses are chilled and kept chilled until they reach the consumer.
- After the ripening process is complete, the vast majority of cheese varieties are also refrigerated.
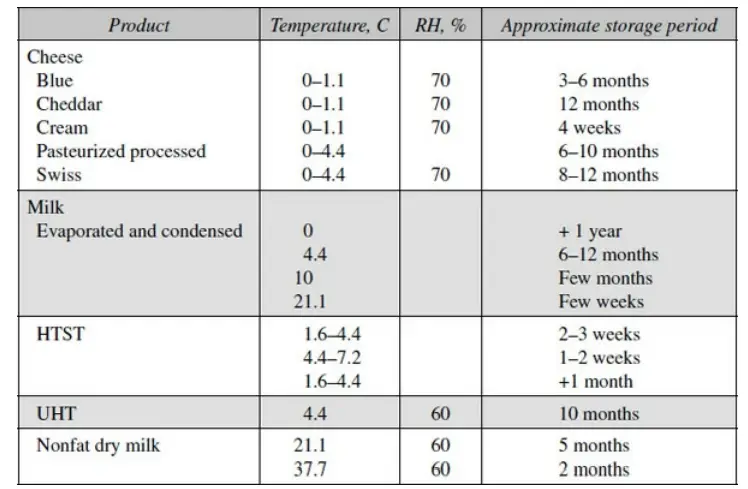
b. Freezing
- As part of the manufacturing process, ice cream and other frozen dairy desserts are frozen and stored at low temperatures in the frozen form, where microbial multiplication is impossible.
- The microbiological composition of the ingredients — milk, cream, sugar, eggs, stabilisers, flavourings, and colorings — and contamination acquired during processing will determine the number and types of bacteria in the mixture and its microbial content after pasteurisation and freezing.
- Pasteurization reduces the number and types of germs, but freezing kills comparatively few organisms, and storage in the frozen condition allows the majority of microbes to survive for extended durations.
- At temperatures of 17 to 18 C or lower, no microbial development is possible in stored butter.
- Significant quantities of frozen cream are stored at a same temperature. Concentrated milk can be frozen at 17 to 18 degrees Celsius and stored at 23 to 24 degrees Celsius or below for several weeks without degrading.
- Freezedrying procedures can concentrate frozen milk. Pasteurized whole milk has been frozen at temperatures between 28 and 29 degrees Celsius and delivered and stored in a frozen state.
5. Drying
Various milk products are created by extracting varying proportions of water from full or skim milk. Only when dry products are manufactured is enough moisture removed to prevent the growth of microbes. In liquid condensed milk products, the reduction in moisture and subsequent increase in the concentration of dissolved chemicals prevents the growth of some types of bacteria.
a. Condensed Products
- Evaporated milk is produced by eliminating approximately 60 percent of the water from whole milk, leaving behind approximately 11.5 percent lactose and double the amount of soluble inorganic salts found in whole milk.
- This high sugar concentration inhibits the growth of some bacteria and may slow or halt the growth of some survivors of the previously outlined heat treatments.
- Bulk condensed milk is more concentrated than evaporated milk, making it a poorer growth medium for microorganisms that cannot tolerate high sugar concentrations.
- Condensed whey, also known as whey semisolids, is another concentrated dairy product, as is condensed buttermilk, also known as semisolid buttermilk, whose acid and other solute concentrations are raised through the condensation process.
- In the preparation of the product from whole milk, sufficient sugar (typically sucrose but occasionally glucose) is added to the milk prior to evaporation to produce sweetened condensed milk with a total average sugar content (lactose plus added sugar) of approximately 54 percent, and in the liquid portion, over 64 percent.
- The product derived from skim milk has approximately 58% total sugar and approximately 66% sugar in the liquid portion of the condensed product.
- These extremely high sugar concentrations and the increased proportion of soluble inorganic salts bind the water, rendering it inaccessible to all but osmophilic microbes.
- Therefore, drying, both by removing water and by wrapping it up, is a primary factor in food preservation. As a result of the can’s evacuation and the aseptic effect of the can’s seal, a product with good keeping quality is produced.
b. Dry Products
- Milk, skim milk, cream, whey, buttermilk, ice cream mix, and malted milk are some of the dairy products available in dry form.
- Since identical concepts generally apply to all of these products, dry skim milk or nonfat dry milk solids will serve as a representative example.
- The majority of dry milk is produced by the roller process, with or without suction, or the spray method.
- Prior to the ultimate drying process, the milk is condensed three to five times for the roller method and two to three times for the spray method. Typically, milk is heated prior to drying (to 65 to 85 C for the roller process and to 68.8 to 93.3 C for the spray process).
- This preheating technique pasteurises the milk and destroys any germs that are less heat-resistant. Modern processing techniques for the production of nonfat dry milk improve circumstances to produce a product with the greatest desirable solubility and flavour.
- Some drying methods include an instantization step in which the dry powder is rewetted and redried. In this procedure, the product is exposed to the atmosphere at nine distinct stages.
- A significant salmonellosis outbreak linked to milk powder was determined to have been caused by air pollution during the instantizing procedure.
- The microbiological composition of a heat-dried dairy product is dependent upon the liquid product to be dried, the preheating temperature and time, the evaporation process (if employed), contamination and growth in storage tanks and pipelines, the drying method, and contamination from air sources.
- Preheating kills microorganisms in the same manner as pasteurisation, eradicating all but thermodurics. Increases in thermoduric and thermophilic microorganisms may be caused by evaporation, particularly if it is a continuous process.
- If the evaporated or concentrated product is stored, the number of microorganisms may rise due to the low temperature of evaporation and the rapid cooling of the milk.
- With the exception of bacterial spores, the high temperature of the roller process without vacuum eliminates nearly all organisms. Without considering the fatal effect of preheating or evaporation and concentration, the actual spray-drying method cannot be relied upon to eradicate germs from the final product.
- In general, thermoduric bacteria, such as heat-resistant streptococci, micrococci, aerobic and anaerobic sporeformers, and microbacteria, are most prevalent in the dry product.
- Multiple incidents of food illness attributable to salmonellae or staphylococci in milk powder demonstrate that these bacteria can sometimes persist in the final product.
6. Use of Preservatives
a. Added Preservatives
- To a limited extent, the addition of preservatives to dairy products is permitted. In cottage cheese, yoghurt, and certain hard cheeses and processed cheeses, the use of sorbic or propionic acid or one of its salts is permissible.
- The purpose of applying a preservative to hard cheeses or preserved cheeses is to prevent the formation of mould on the surface.
- Similarly, adding preservatives to cottage cheese and yoghurt prevents the growth of moulds on the product’s surface and extends its shelf life.
- Sugar added to sweetened condensed milk functions as a preservative; it reduces the aw, making moisture unavailable to microorganisms.
- In the production of various types of cheese, sodium chloride or table salt is typically added more for flavour or for suppressing the growth of microbes during production and curing than for preservation of the final product.
- In salted butter, the concentration of sodium chloride in the liquid phase is sufficient to inhibit the growth of several bacteria and reduce the number of those that are intolerant to salt.
- The aqueous phase of butter with 16.34 percent moisture and 2.35 percent salt would include approximately 12.6 percent salt brine.
- Cheese is smoked largely for flavour enhancement, however the drying, especially of the rind, and the chemical preservatives produced by the smoke may extend its shelf-life.
- Mold growth is typically delayed or stopped when sorbic acid, sorbates, propionic acid, or propionates are added to or incorporated into cheese packaging.
- For the pasteurisation of milk for specific types of cheese, hydrogen peroxide has been mixed with a modest heat treatment (e.g., Swiss and Cheddar). Typically, excess peroxide is neutralised by the use of catalase.
b. Developed Preservatives
- The majority of fermented products are microbiologically more stable or have a longer shelf life than the starting material.
- Therefore, fermented milk and cheese have a longer shelf life than fluid milk due in part to the acidity produced by the bacterial growth.
c. Other Methods
- Although milk can be treated with ultraviolet radiation to achieve an effect equal to pasteurisation, this approach is not utilised to preserve milk since only a thin layer of milk can be properly irradiated, and a “burnt” flavour would develop if care is not followed.
- Other applications of ultraviolet light in the dairy sector include the irradiation of rooms to minimise the quantity of microorganisms in the air in processing rooms where sweetened condensed milk is manufactured or sliced cheese is packaged, as well as in cheese-curing rooms.
- On the sides of the cheese exposed directly to the rays, mould growth is inhibited, but not on the shadowed side. Ultraviolet light is also used to prevent mould formation on the surface of storage tanks storing liquid sugar or corn syrup solids (ingredients in ice cream).
- Milk and its products are preserved through the use of multiple preservative agents. Milk for market sale or dairy product manufacturing is prepared as aseptically as possible, cooled quickly, and kept refrigerated.
- It is pasteurised and packaged to prevent the growth of bacteria. Cream is similarly processed. Fermented milks owe the majority of their preservation abilities to the acid produced during fermentation, but they must be chilled and packaged for storage.
- Primarily, butter is maintained by low temperatures, cooling for short-term storage or freezing for long-term preservation.
- In addition to the low moisture and salt content, packaging or sealing also aids in preventing contamination. Cheese is maintained by the acidity created during its production, by chilling, by its impermeable rind, or by packaging.
- Properly prepared dry milk has little moisture for microbial growth, but it must be packaged to prevent contamination.
- Milk that has been evaporated is treated with steam under pressure to eliminate all or the majority of bacteria and then sealed in cans to prevent contamination.
- During its preparation, sweetened condensed milk is pasteurised, includes a high concentration of sugars, and is protected by a sealed can.
7. Irradiation
- The emission and spread of energy across space or a medium constitutes radiation.
- Ionizing radiations are of significant concern when it comes to food preservation. IR is described as radiations with wavelengths of 2000 nanometers or less, such as a-particles, b-rays, X-rays, and cosmic rays.
- Radiations kill microorganisms in foods by direct or targeted mechanisms. In this process, it is thought that a cellular or subcellular entity induces death when struck by a single high-energy particle.
- Radiation has diverse impacts on cells, and some damage can be repaired. The most vulnerable food to irradiation is milk.
- Therefore, g-irradiation of milk below the permissible limit of 1 M rad results in off-flavors such as malty, caramel, cooked, burnt, oxidised, cabbage, and rotten.
- It has also been discovered that g-irradiation negatively affects the vitamin content of a number of foods.
- In numerous tests on cow, buffalo, and goat milk, a dose-dependent increase in total plate count and spore-forming organisms was seen, although coliforms, yeasts, and moulds were reported to be entirely eradicated.
- The microbiological grading of milk has improved significantly, as determined by dye reduction tests, without compromising its titratable acidity or pH.
8. Sterilisation
A sterile product is one that has no live germs. Temperatures slightly above the maximum for bacterial growth result in the death of vegetative bacterial cells, while spores can withstand much higher temperatures and are hence the primary concern in the majority of sterilisation methods. The initial method of sterilisation, which is still in use, is in-container sterilisation, which typically takes 20 to 30 minutes at 115 to 120 degrees Celsius. After fat standardisation, homogenisation, and heating to around 80 °C, milk is often packaged in glass or plastic bottles for milk and cans for evaporated milk. In batch production, the product is moved to autoclaves while in continuous production, it is transferred to a hydrostatic tower.
UHT treatment
The acronym UHT stands for Ultra High Temperature. UHT treatment is a method for preserving liquid food products by subjecting them to brief, high heating, typically between 135 and 140 degrees Celsius. This eliminates the microorganisms that might otherwise ruin the goods. Continuously occurring in a closed system, UHT treatment protects the product from becoming contaminated by airborne microorganisms. The product rapidly transitions between heating and cooling phases. To prevent reinfection of the product, aseptic filling is a vital aspect of the procedure. Two alternate UHT treatment procedures include
- indirect heating and cooling through heat exchangers.
- Direct heating via injection of steam or infusion of milk into steam, and cooling by expansion under vacuum conditions.
Spoilage of Milk and Milk Products
As previously stated, milk and milk products are preserved in a variety of ways, some of which include killing only a portion of the bacteria present and slowing their growth. Therefore, some products have a limited shelf life, and many perish quickly if poor preservation procedures are employed.
1. Milk and Cream
- Milk is an ideal growth medium for numerous types of bacteria due to its high moisture content, relatively neutral pH, and plenty of microbial food sources.
- There is an abundance of food for energy in the form of lactose, butterfat, citrate, and nitrogenous substances (proteins, amino acids, ammonia, urea, and other compounds), as well as the necessary accessory foods and minerals for bacteria.
- Some inhibitory chemicals (lactoperoxidase and agglutinins) are present in freshly obtained milk, but their effectiveness diminishes rapidly.
- Due to the fermentable sugar, an acid fermentation by bacteria is most likely in raw milk under normal conditions; nevertheless, additional modifications may occur if conditions are hostile to the acid formers or they are absent.
- With a tendency toward greater pasteurisation temperatures, the spoiling flora of pasteurised milk is increasingly comprised of heat-resistant, spore-forming, psychrotrophic bacilli.
- When milk becomes sour, it is typically considered spoilt, particularly if it curdles. The initial sign of acid development is a sour taste, followed by the coagulation of milk into a solid curd or a weaker curd that discharges clear whey.
- Most likely to undergo lactic acid fermentation is raw milk stored at room temperature.
- Streptococcus lactis is most likely to induce sourness in raw milk at temperatures between 10 and 37 degrees Celsius, with possible growth of coliform bacteria, enterococci, lactobacilli, and micrococci*. At higher temperatures, such as between 37 and 50 degrees Celsius, S. thermophilus and S. faecalis may create approximately 1 percent acid, followed by lactobacilli such as Lactobacillus bulgaricus*, which produce more acid.
- Some lactobacilli can grow at temperatures exceeding 50 degrees Celsius, but produce less acid. Thermophilic bacteria, such as L. thermophilus*, may grow at even greater temperatures. There is little acid production in milk stored at temperatures close to freezing, however proteolysis may occur.
- Pasteurization of milk eliminates the more active acid-forming bacteria but may allow the survival of heat-resistant lactics (e.g., enterococci, Streptococcus thermophilus, and lactobacilli), which will result in lactic acid fermentation if the subsequent storage temperature is high enough.
- If conditions are unfavourable for lactic acid bacteria, several bacteria other than lactics can induce an acid fermentation in milk.
- The coliform bacteria create a little quantity of lactic acid as well as significant quantities of volatile byproducts, including hydrogen, carbon dioxide, acetic acid, formic acid, alcohol, etc.
- Micrococcus*, Microbacterium, and Bacillus species can create acid in milk, predominantly lactic acid, but cannot normally compete with the lactics. Clostridium spp. can create butyric acid in milk under conditions that prohibit or hinder the typical development of lactic acid.
- Thus, after a heat treatment that eliminates all bacterial vegetative cells but permits Clostridium spores to survive, milk may undergo butyric acid fermentation, resulting in the formation of hydrogen and carbon dioxide gas.
a. Gas Production
- Almost always accompanied by acid formation, gas production by bacteria is generally undesirable in milk and milk products.
- Principal gas-producing organisms include coliform bacteria, clostridium spp., gas-producing Bacillus species that produce both hydrogen and carbon dioxide, and yeasts, propionics, and hetero-fermentative lactics that only produce carbon dioxide.
- The production of gas in milk is indicated by foam on the surface if the milk is liquid and supersaturated with the gas, by gas bubbles trapped in the curd or furrowing it, by floating curd containing gas bubbles, or by a tearing apart of the curd caused by rapid gas production, resulting in the so-called stormy fermentation of milk.
- The likelihood of gas formation and the microorganisms responsible depend on the milk’s pretreatment and holding temperature. Coliform bacteria are most likely to be the primary gas-producing organisms in raw milk.
- Heterofermentative lactics may also produce gas, but typically not enough for the milk to be noticeable. Yeasts (lactose-fermenting) are either missing or present in low levels in milk and compete poorly with bacteria.
- Gas-forming At higher temperatures, Clostridium and Bacillus do not compete well with acid formers, but they can operate if the acid formers are absent or relatively inactive.
- Thus, in milk cooked to pasteurisation temperatures or above, the principal acid-forming organisms will be destroyed, while Clostridium and Bacillus spores will survive and sporeformers may produce gas.
- Propionic acid-forming bacteria are inactive in milk but produce carbon dioxide gas in cheese, as will be detailed further on.
b. Proteolysis
- Some of the peptides that are released during the hydrolysis of milk proteins by microorganisms impart a bitter flavour to the milk.
- Storage at a low temperature, the destruction of lactics and other acid formers by heat, and the destruction of formed acid in the milk by moulds and film yeasts or the neutralisation of acids by products of other organisms all contribute to the acceleration of proteolysis.
- Changes caused by proteolytic microorganisms include (1) acid proteolysis, in which acid production and proteolysis occur simultaneously, (2) proteolysis with little acidity or even alkalinity, (3) sweet curdling, which is caused by renninlike enzymes of the bacteria at an early stage of proteolysis, (4) slow proteolysis by intracellular enzymes of bacteria after their autolysis, and (5) residual proteolytic activity of heat-stable proteinase.
- Pseudomonas fluorescens, for instance, produces a proteinase that can survive pasteurisation even though the bacterium itself cannot. Acid proteolysis results in the formation of a shrunken curd and the release of a great deal of whey.
- This is followed by the curd’s slow digestion, during which its appearance changes from opaque to translucent and it may be completely dissolved by certain types of bacteria.
- Occasionally, distinct curd particles are formed that shrink so much that they are barely discernible in the vast quantity of whey. Several species of Micrococcus*, some of which grow in the udder of the cow and cause acid proteolysis in aseptically withdrawn milk, can cause acid proteolysis.
- Streptococcus faecalis var. liquefaciens*, one of the intestinal streptococci or enterococci, is a lactic acid organism that is also actively proteolytic. As with other enterococci, it is thermoduric and may cause acid proteolysis in pasteurised milk.
- Species of Bacillus that contain lactose-fermenting, proteolytic strains can survive pasteurisation or a more rigorous heat treatment of milk and cause acid proteolysis.
- Proteolysis by bacteria unable to ferment lactose varies depending on the bacterium involved, ranging from casein digestion to proteolysis that is only detectable through a chemical test.
- The majority of these bacteria produce negligible acidity; in fact, milk typically becomes alkaline over time due to the breakdown of proteins.
- Many of these bacteria “sweet-curdle” the milk (coagulation with little acid) prior to digesting the casein, whereas others hydrolyze the protein so rapidly that there is no curdling and a relatively clear liquid is left with no trace of casein or curd.
- Actively proteolytic bacteria are found in Micrococcus*, Alcaligenes, Pseudomonas, Proteus, Flavobacterium, and Serratia, which are all genera of non-spore-forming bacteria, as well as Bacillus and Clostridium, which are genera of spore-forming bacteria.
- Some Micrococcus*, Pseudomonas, Alcaligenes*, Flavobacterium, and Bacillus species can grow at low temperatures and are therefore likely to cause proteolysis and/or bitterness in chilled milk.
- Bacillus cereus has been linked to the formation of sweet curds in pasteurised milk. This condition is becoming increasingly prevalent in milk as a result of (1) increased pasteurisation temperatures, (2) the psychrotrophic capacity of some bacilli, and (3) increased holding or shelf-life durations.
- Coagulation or curd development typically begins at the bottom of the carton and may not be noticeable to the consumer at first.
- Slow proteolysis by intracellular enzymes of bacteria following their autolysis is not substantial in milk under normal conditions, but it is relevant when a long time is permitted for their work, such as when ageing cheese or storing long-lasting products.
c. Ropiness
- Ropiness and sliminess can occur in milk, cream, and whey, but they are most significant in commercial milk and cream.
- Nonbacterial ropiness or sliminess may be caused by (1) stringiness caused by mastitis and in particular by fibrin and leukocytes from the cow’s blood (unlike ropiness produced by bacteria, it is present when the milk is drawn and does not develop during holding of the milk), (2) sliminess resulting from the thickness of cream, e.g. at the top of a bottle, or (3) stringiness due to thin films of casein or lactalbumin during cooling, as is sometimes observed
- This is a transitory impact. Bacterial ropiness is induced by slimy capsular material from the cells, typically gums or mucins, and develops optimally at low storage temperatures.
- Generally, ropiness reduces as the acidity of milk or cream rises. There are two primary varieties of bacterial ropiness, one in which the milk is ropiest at the top and the other in which the milk is ropiest throughout.
- The most common source of surface ropiness is Alcaligenes viscolactis, a primarily aquatic or soil-dwelling organism that grows reasonably well in the neighbourhood of 10 degrees Celsius. Some thermoduric micrococci, such as Micrococcus freudenreichii*, can induce ropiness on the skin’s surface. Several types of bacteria are capable of causing ropiness throughout the milk:
- Enterobacter aerogenes, Enterobacter cloacae, Klebsiella oxytoca, and Escherichia coli seldom. Enterobacter-caused lethargy is typically worse at the top of the milk.
- Various strains of certain prevalent species of lactic acid bacteria. Streptococcus lactis var. hollandicus induces ropiness in milk and is used to produce fermented milk in Scandinavia. Lactobacillus casei, L. bulgaricus*, and L. plantarum, as well as Streptococcus cremoris strains, can infrequently cause ropiness. It is believed that the ability of the majority of these lactic bacteria to form long chains adds to the stringy nature of milk.
- In addition to alkali formers, micrococci*, streptococci, and bacilli, there are various additional bacteria. Typically, the acid formers would restrict these microorganisms.
- Since water, manure, utensils, and feed are the sources of the germs that cause ropiness, reducing or eliminating contamination from these sources helps avoid ropiness. Milk pasteurisation effectively eliminates the vast majority of these types of bacteria.
d. Changes in Milkfat
Milkfat can be broken down by several bacteria, yeasts, and moulds that do not form different groups based on other features. The majority of the bacteria are aerobic or facultative, proteolytic, and acid-free. The following modifications occur in the milkfat:
- Oxidation of the unsaturated fatty acids produces aldehydes, acids, and ketones, resulting in tallow-like aromas and flavours. The process is promoted by metals, sunshine, and microorganisms that oxidise.
- The enzyme lipase converts butterfat into fatty acids and glycerol. The lipase could have been present in the original milk or it could be microbial.
- Oxidation and hydrolysis combined to cause rancidity.
Numerous bacterial genera contain lipase-producing bacteria, including Pseudomonas, Proteus, Alcaligenes, Bacillus, Micrococcus*, Clostridium, and others. The majority of moulds and some yeast species are lipolytic. Pseudomonas fragi and Staphylococcus aureus produce relatively heat-resistant lipases that, if present in raw milk, may survive pasteurisation.
e. Alkali Production
- There are bacteria in the group of alkali formers that generate an alkaline response in milk without any signs of proteolysis.
- The alkaline reaction may be caused by the creation of ammonia, as in the case of urea, or carbonates, as in the case of organic acids such as citric acid.
- The vast majority of these bacteria thrive at moderate to low temperatures, and many are resistant to pasteurisation. A. viscolactis and Pseudomonas fluorescens are examples of alkali formers.
f. Flavor changes
The flavour of freshly drawn milk is mild, delicate, and easily modified. Since individuals vary in their capacity to discern flavours and in their descriptions of them, a number of names that are more or less descriptive have been proposed to describe off-flavors. As extracted from the cow, milk may have an odd flavour due to the individual cow, mastitis, the lactation stage of the cow, or the diet. The following are some of the off-flavors created by microorganisms:
- Sour or Acid Flavor: The acidity can be described as “clean” when it is produced by Streptococcus lactis and other lactics; as “aromatic” when lactic streptococci and aroma-forming Leuconostoc species are growing together; and as “sharp” when appreciable amounts of volatile fatty acids (formic, acetic, or butric) are produced by coliform bacteria, Clostridium spp., and In fermented milk products, clean and fragrant aromas are sought, but harsh flavours are undesired.
- Bitter Flavors: Bitterness typically originates from proteolysis, but it can also result from lipolysis or even lactose fermentation. Occasionally, milk from cows late in their lactation cycle is slightly bitter. In a previous paragraph, microorganisms responsible for proteolysis and consequently bitterness were discussed. Additionally, certain strains of coliform bacteria and asporogenous yeasts can cause bitterness. Other cocci cause extremely bitter milk, and some actinomycetes cause bitter, musty tastes.
- Burnt or Caramel Flavor: Certain strains of Streptococcus lactis var. maltigenes produce this flavour, which is similar to the cooked flavour of hot milk.
- Miscellaneous Other Tastes: Other uncommon flavours comprise a lengthy list, of which just a portion will be mentioned: a barny flavour by Enterobacter oxytocum*; soapiness by ammonia formers such as Pseudomonas sapolactica*; a turniplike flavour by Escherichia coli and P. fluorescens; a malty flavour by yellow micrococci from the udder; a fruity flavour by Pseudomonas fragi; a potatolike flavour by Pseudomonas Other flavours have been described as filthy, stale, astringent, oily, herbaceous, carrot-like, etc. Raw milk that has been improperly chilled and stored in a securely sealed container so that volatile byproducts of bacterial metabolism have accumulated on the surface of the milk emits an odour that varies in character. This situation is known as smothered milk.
g. Color Changes
The colour of milk or cream is influenced by its physical and chemical makeup, such as the quantity and yellowness of the butterfat, the milk’s consistency, the presence of blood and pus, and the animal’s diet. Alterations in colour can be induced by microorganisms in addition to the aforementioned changes, but there are a few colour changes that deserve special note. The colour may result from the formation of coloured bacteria or moulds on the milk’s surface in the form of a scum or ring, or it may be present throughout the milk.
- Blue Milk: In pure culture, Pseudomonas syncyanea generates a bluish-gray to brownish colour in milk, however growth alongside an acid former such as Streptococcus lactis results in a deep blue colour. This deficiency and the blue hue produced by actinomycetes or Geotrichum species are uncommon.
- Yellow Milk: Pseudomonas synxantha* may be responsible for the yellowing of the cream layer of milk, which coincides with lipolysis and proteolysis. Flavobacterium species can also cause yellowing.
- Red Milk: Red milk is typically caused by Serratia species, such as S. marcescens, but is uncommon since other bacteria typically outcompete the red-pigmented species. Brevibacterium erythrogenes* creates a red coating on the surface of milk, which is thereafter degraded by proteolysis. On the surface of sour milk or cream, Micrococcus roseus may develop and form a crimson residue, and yeast may produce pink or red colonies. Blood will impart a red hue to milk. Red blood cells can settle or be separated by centrifugation.
- Brown Milk: A brown hue may result from Pseudomonas putrefaciens* or P. fluorescens’ enzymatic oxidation of tyrosine.
Spoilage of Milk at DifferentTemperatures
At any given storage temperature, the majority of raw milk samples follow a predictable sequence of microbial-induced alterations. At refrigeration temperatures, psychrotrophic bacteria such as Pseudomonas may induce proteolysis, leading to the development of moulds. At room temperature, lactic streptococci and coliform bacteria are most likely to initiate acid fermentation, followed by acid-tolerant lactobacilli. Then, moulds or film yeasts on the surface reduce the acidity, allowing additional acid to develop. Proteolytic or putrefactive bacteria complete the breakdown once the majority of the acid has been neutralised. Pasteurization as used commercially in HTST systems eliminates yeasts, moulds, the majority of psychrotrophic bacteria, coliforms, and fast acid makers such Streptococcus lactis. Consequently, the spoiling of pasteurised milk depends on:
- The “thermoduric” and spore-forming bacteria that survive pasteurisation.
- The bacteria that enter the milk after pasteurisation, postpasteurization contamination from equipment and the filling process, and the packaging itself.
- The potential presence of leftover heat-resistant microbial enzymes.
- The storage area’s temperature.
Obviously, the microbial flora is established once the milk is pasteurised and sealed in the carton, unless the carton loses its integrity. Consequently, the storage temperature determines which organism will predominate and the pace of decomposition for each carton. Similarly, the initial number and types of organisms present after pasteurisation affect the pace of spoiling if the storage temperature remains constant.
Since milk is chilled, psychrotrophic microbes typically cause deterioration. At low temperatures, growth is gradual but substantial. Assuming an 8-hour generation time at 7 degrees Celsius, 1 cell might become 1 million in 20 generations, or around 6 to 7 days; 2 million in 7 to 8 days; and 1 billion in 10 days.
It is plausible to expect extremely heat-resistant psychrotrophic organisms to be an issue in pasteurised milk, given the superb refrigeration to which milk (raw and pasteurised) is currently subjected and the ongoing trend toward higher pasteurisation temperatures. Psychrotrophic bacilli that generate spores are the cause of the current difficulties with the spoiling of pasteurised milk.
Spoilage of Condensed and Dry Milk Products
- This section contains evaporated milk (unsweetened), condensed milk in bulk, frozen milk, sweetened condensed milk, condensed whey or buttermilk, condensed sour skim milk, and dry milk.
- All of these products’ quality depends on the quality of the condensed or dried raw material, as flaws in the raw material are transferred to the condensed or dried product.
- All condensed products have a reasonably high concentration of solutes, which prevents the growth of certain bacteria.
- Dry milk is so low in moisture that, when handled properly, it poses no microbiological spoiling issues. Mold may grow if the relative humidity is greater than 8 percent.
- Molds are responsible for the only deterioration of condensed buttermilk and sour skim milk when exposed to air. The high concentration of acid and solutes inhibits bacterial and fungal growth.
Bulk Condensed Milk
- The forewarming temperatures utilised in the production of simple condensed milk are merely comparable to pasteurisation, and the evaporation process occurs at a temperature low enough to allow the formation of thermophiles.
- Therefore, despite being chilled, this product has a short shelf life and is susceptible to deterioration by thermoduric bacteria that are tolerant of the increased solute concentration in the condensed product.
- In superheated condensed milk, the milk’s temperature is elevated to 65,6 to 76,7 degrees Celsius by the addition of steam, a method that likely eliminates the majority of bacteria’s vegetative cells but not their spores. This item also has a short shelf life.
Evaporated Milk
- In an effort to kill all microorganisms, unsweetened evaporated milk is canned and treated with heat and steam pressure.
- Only when the heat process is inadequate or when flaws in the container allow microorganisms to enter may food spoil.
- Surviving bacterial spores may induce can swelling, milk clotting, or the development of an unpleasant flavour.
- Gas-forming anaerobic sporeformers (Clostridium) are the primary cause of the can’s expansion, however overfilling the can with cold milk can also cause expansion.
- Long-term storage may cause the can to bulge as a result of acidic milk ingredients reacting with the iron of the can to create hydrogen gas. The milk in the container may contain anywhere from a few flakes to a full curd.
- Bacillus species are typically to blame, including mesophiles such as B. cereus, B. subtilis, and B. megaterium, a facultative thermophile, B. coagulans, and an obligate thermophile, B. calidiolactis*.
- The amount of curd in the milk is somewhat determined by the amount of air in the can. Thermophilic spoilage should not be a problem if the milk is chilled quickly and maintained cool, but it can be a problem in the tropics.
- Bitterness is typically caused by the proteolysis of Bacillus and, less frequently, Clostridium species.
- In rare situations, some of the latter may cause putrefaction. Leakage-caused spoilage, as indicated by the presence of nonsporeformers, may result in gas and swelling due to coliform bacteria or yeasts, coagulation due to streptococci, or bitterness due to cocci.
Sweetened Condensed
- Milk Yeasts, moulds, and the majority of the vegetative cells of bacteria are killed by subjecting sweetened condensed milk to temperatures between 71.1 and 100 degrees Celsius during forewarming and temperatures between 48.9 and 54.4 degrees Celsius during condensing.
- In addition, around 55 to 60 percent of the total sugar is sugar (lactose plus added sugar). Additionally, the can is emptied and shut.
- Therefore, spoilage is primarily caused by organisms that have infiltrated the food after heat treatments, particularly if air is present.
- The most common types of spoiling are (1) gas formation caused by sucrose-fermenting yeasts or, less frequently, by coliform bacteria, (2) thickening induced by micrococci, which likely generate renninlike enzymes, and (3) “buttons,” which are mould colonies forming on the surface of milk.
- The amount of air in the container determines the size of these buttons. Aspergillus and Penicillium species, such as A. repens, have been implicated.
Spoilage of Frozen Desserts
- Ice cream, ice milk, frozen custards, sherbets, and ices are frozen sweets. Milk, cream, evaporated milk, condensed milk, dried milk, colouring materials, flavours, fruits, nuts, sweetening agents, eggs and egg products, and stabilisers may be present in various combinations.
- Any of these may contribute germs to the product and impair the dessert’s quality as measured by its bacterial content or the presence of specific types of bacteria, such as coliforms.
- However, as long as the desserts are maintained frozen, they are often not susceptible to spoiling.
- The only significant types of spoiling occur in the ingredients before to mixing or in the mixture prior to freezing.
- Since the mixture is pasteurised before it is frozen, there should be no spoiling issues unless it is kept at temperatures above freezing for an extended period of time, at which point acid-producing bacteria may cause it to become rancid.
Spoilage of Butter
- Many of the flaws in butter originate in the cream from which it is produced, particularly if the cream has been stored on the farm for several days prior to being collected by the creamery.
- During this period, lactic acid bacteria, gas-producing organisms, and other spoiling organisms may proliferate, followed by moulds such as Geotrichum candidum.
- Infrequently occurring lactose-fermenting yeasts may cause excessive gas pressures in the can of cream. In reality, the majority of the types of spoilage discussed for milk at the beginning of this chapter are also capable of occurring in cream and affecting butter produced from it.
- The likelihood and nature of spoiling will depend on the type of butter and the storage environment.
- Due to the high salt concentration in the tiny quantity of moisture present, salted butter is less likely than unsalted butter to enable microbial development. Generally, sweetcream butter has a longer shelf life than sour cream butter.
- The majority of butter produced in the United States today is manufactured from pasteurised cream, in which the majority of spoilage organisms have been eliminated.
- In addition, butter is typically chilled, and during commercial storage, it is kept at a temperature of 17.8 degrees Celsius, where no microbial development may occur.
- Due to these factors, bacteria rarely thrive in butter, and when they do, their growth is typically limited.
- However, because the flavour of good butter is so delicate, relatively tiny levels of growth can cause significant flavour loss.
Flavor Defects
As previously said, unwanted flavours may originate from the cream, which may acquire them through the cow’s diet, absorb them from the surroundings, or produce them through microbial growth. Off-flavors are contributed to the cream by feeds such as onions, garlic, French weed, peppergrass, and subpar silage. Volatile items that can be absorbed from the air include barn scents and chemicals used in the barn, such as kerosene, gasoline, insecticides, disinfectants, etc. The growth of microorganisms in cream and the milk from which it is separated can cause any of the following undesirable flavours:
- Lactobacilli-driven cheesiness
- Lipolytic bacteria and moulds, and maybe lipase in the cream, cause rancidity.
- Barny flavour provided by Enterobacter species.
- Streptococcus lactis var. maltigenes* produces a malty flavour.
- Yeast-produced flavour with a yeasty taste.
- Molds and actinomycetes contribute to musty aromas.
- Dissolved metals in a highly acidic cream create metallic tastes.
- Flavorlessness arising from the degradation of diacetyl by bacteria such as Pseudomonas.
- Extremely acidic taste, when the cream contains high acidity.
- Coliform bacteria provide a “dirty” flavour Unsatisfactory processing procedures may result in a cooked flavour from over-pasteurization of the cream of a “neutralizer” flavour if too much of the neutralising chemical is employed, if it is distributed unevenly in the cream, or if pasteurisation occurs before the proper balance is established.
Similar to cream, butter quickly absorbs volatile airborne substances. Microorganisms in butter can result in the following flaws:
- Surface taint, also known as “rabbito” and “putridity,” is attributed to Pseudomonas putrefaciens and is typically introduced via wash water, churns, or machinery. In unsalted or low-salt butter, it is worse. The “sweaty-foot” stench is mostly caused by isovaleric acid.
- Aeromonas hydrophila induces a fishy taste.
- The action of P. fragi generates esters-like flavours.
- P. mephitica produces aromas reminiscent of skunk.
- Roquefortlike flavours, generated by moulds.
Chemically produced flavours include (1) rancidity caused by lipase in the cream, (2) tallowiness caused by oxidation of unsaturated fats catalysed by copper and bacterial enzymes and favoured by a low pH, low-temperature pasteurisation, salt, air, and ozone, and (3) fishiness caused by the production of trimethylamine from lecithin. High acidity, salt overwork of the butter, and the presence of copper enhance this flaw.
Color Defects
- Not caused by microorganisms are mottling due to inappropriate operation, a pink hue generated by the sulphur dioxide refrigerant on the butter colour, surface darkening caused by the loss of water from surface layers, and bleaching caused by tallowiness.
- Molds, yeasts, or bacteria that originate from churns, wrappers, liners, circles, tubs, the air, or unpasteurized cream can cause primarily surface discolorations.
- Colored mould growths produce a smeared or Alternaria-type discoloration, with dark, smoky, or (occasionally) greenish regions where Alternaria or Cladosporium species have formed, or little black spots of Stemphylium.
- Penicillium produces a green hue, whilst Phoma or Alternaria generates brown spots, and Geotrichum (synonymous with Oospora) produces orange or yellow dots.
- Fusarium culmorum can generate brilliant reddish-pink patches. Sometimes yeasts grow in pink colonies. Pseudomonas nigrifaciens is responsible for the reddish-brown discoloration in lightly salted butter.
Prevention Of Contamination Before Processing
1. Clean Milk Production
Producing milk with fewer bacteria is one of the most effective methods for preventing the deterioration of milk and milk products. When the udder secretes milk, it is nearly sterile. To achieve this objective, the following clean milk production strategies can be implemented:
- During the milking process, care must be taken to maintain cleanliness and completeness.
- Antibiotic infusion of the udders of all milk-producing animals prior to drying off and of udders exhibiting clinical symptoms of mastitis during lactation.
- Before and after milking, the teats and udder must be washed with a disinfectant solution and dried.
- The feeding of roughage during milking should be avoided. Milker should be in good health, wear clean and snug clothing, routinely cut his nails, wash and dry his hands before milking, and avoid sneezing and coughing during milking.
- Preventing dust particles from falling into the milk pail.
- Using potable water to clean animals and milking equipment.
- The Acid Boiling Water (ABW) System is used to clean the milking machine pipelines (Boiling water is sucked through the plant by the vacuum, adding acid in the first 4 min. of flow and washing acid free in the remaining 2 min).
- Effective cleaning of equipment and containers, such as the milk pail, milking machine, teat dip, clusters, cans, milk pipeline, recorder, bulk tank, strainer, cooler, and milk flow indicators, in order to prevent the deposition of leftover milk solids.
- Low storage temperature and maintenance of hygienic conditions for storage and transport equipment.
2. Prevention of Microbial Adhesion
- Food-contact surfaces must be kept clean in order to prevent the establishment of resistant microbial strains.
- When fractures and fissures prevent adhered cells from being removed during cleaning, the microtopography of a surface can sometimes have a negative effect on the cleaning procedure.
- Therefore, good equipment design is a prerequisite for avoiding cracks and dead space known to harbour organic matter. Glass, stainless steel, aluminium, and rubber are employed in the construction of dairy equipment.
- The smooth and corrosion-resistant surface of glass makes it the material of choice for the manufacture of equipment.
- Stainless steel is resistant to impact but susceptible to corrosion, whereas rubber surfaces are susceptible to deterioration and may develop surface fissures that can harbour bacteria.
- Surface cleanliness is another criterion. If cleaning processes result in topographical defects on a surface, it will provide a greater number of attachment sites for microbial growth.
- Sanitizer use might potentially result in surface corrosion. To prevent microbial adherence, these considerations must be taken into account when building food-contact equipment.
- The attachment of bacteria to a surface may result in the production of biofilm. During the first phases of biofilm formation, a conditioning layer is formed due to the absorption of surface proteins.
- Gram-negative bacteria are more adherent to glass than gram-positive bacteria, and their biofilm population after two days of culture is greater than that of gram-positive bacteria.
- The adsorption of a bioactive substance onto a clean food contact surface can be viewed as a potential approach to inhibit the first adherence of microorganisms. On model food contact surfaces, surfaces with the adsorbed antimicrobial peptide nisin reduce the incidence of surface contamination by L. monocytogenes.
- Pathogens, such as S. aureus, S. macescens, and L. monocytogenes, are significantly less likely to adhere to a stainless steel surface that has been pretreated with skim milk.
- Pretreatment with individual milk proteins a, ß, and k reduces attachment as well. It is believed that the release of atomic nitrogen by these proteins is responsible for this antibacterial activity.
- The ultrasonic treatment can be used to disperse clumps of microorganisms in order to promote their eradication by later bactericidal treatment with detergents and sanitizer.
Milk Pasteurization
The name “pasteurisation” honours Louis Pasteur, who in the middle of the 19th century conducted groundbreaking research on the fatal effect of heat on microbes and the application of heat treatment as a method of food preservation. The pasteurisation of milk is a unique form of heat treatment, which can be defined as “any heat treatment of milk that guarantees the annihilation of tubercle bacillus (Mycobacterium tuberculosis) without significantly altering the milk’s physical and chemical qualities.
” Phosphatase detection can be used to determine the effectiveness of pasteurisation. This enzyme is always present in unpasteurized milk and is eliminated by the temperature and time required for effective pasteurisation. All common pathogens likely to be present in milk are destroyed by relatively modest heat treatment, which has a negligible impact on the physicochemical qualities of milk.
The tubercle bacillus (Mycobacterium tuberculosis) is considered to be killed by boiling milk to 63 degrees Celsius for 10 minutes. By heating milk to 63 °C for 30 minutes, total safety can be guaranteed. This pathogen is consequently regarded as the indicator organism for pasteurisation: any heat treatment capable of eliminating it may be depended upon to eliminate all other milk-borne pathogens. In addition to harmful germs, milk includes additional chemicals and microbes that might affect the flavour and shelf life of various dairy products.
Therefore, a secondary function of heat treatment is to eliminate as many of these other species and enzyme systems as possible. This necessitates a more severe heat treatment than is required for pathogen eradication. The combination of temperature and holding time is crucial, as it dictates the heat treatment’s intensity. Tuberculosis bacilli are more heat-resistant than coliform bacteria. To achieve complete destruction, a holding duration of 20 seconds at 70 °C or around 2 minutes at 65 °C is required.
LTLT pasteurization
- Original heat treatment consisted of heating milk to 63 degrees Celsius in open vats and holding it at that temperature for 30 minutes.
- This technique is known as the holder method or the low temperature long time (LTLT) technique.
- Milk is nearly always treated with heat in continuous processes such as thermisation, HTST pasteurisation, and UHT treatment.
HTST pasteurization
- HTST is an acronym for High Temperature Short Time. The actual time/temperature combination varies according on the quality of the raw milk, the type of product being processed, and the desired shelf-life.
- The HTST method for milk entails heating it to 72 to 75 °C for 15 to 20 seconds before cooling it. The phosphatase enzyme is damaged by this combination of time and temperature.
- Therefore, the phosphatase test is performed to ensure that milk has been adequately pasteurised. The test result must be negative; phosphatase activity must not be evident.
Ultra pasteurization
- When a specific shelf life is necessary, ultra-pasteurization might be employed. For some manufacturers, two additional days is sufficient, while others aim for an additional 30 – 40 days on top of the conventional 2 – 16 days associated with pasteurised products.
- The key premise is to decrease the primary sources of reinfection of the product during processing and packaging in order to increase the product’s shelf life. This demands an exceedingly high level of production cleanliness and a temperature for distribution.
- The process of heating milk to 125–138°C for 2–4 seconds and then chilling it to 7 °C is the basis for extending its shelf life. Extended Shelf Life (ESL) is an umbrella term for heat-treated items that have been endowed with increased keeping qualities.
- However, ESL items must be refrigerated throughout distribution and at retail outlets.
Food-borne pathogens and food poisoning in milk production source.
| Mammary gland health status | Cow herd health status | Production environment | Production land water source |
| S. aureusStreptococcus agalactiaeStreptococcus spp.Streptococcus pyogenesStreptococcus zooepidemicus(B-hemolytic Streptococcus Lancefield C group) Corynebacterium ulcerans | Mycobacterium bovisMycobacterium avium subsp. paratuberculosisBrucella ssp.S. aureus MRSA-LASalmonella typhimurium phage type 561 (STM DT7) | Listeria monocytogenes Salmonella ssp.E. coli O 157:H7E. coli (STEC)E. coli (EHEC)Yersinia enterocolitica Enterobacter sakazakiiCampylobacter jejuni Enterococcus faecalis Citrobacter freundiiBacillus cereus+Cryptosporidium parvumCoxiella burnetii*Toxoplasma gondii* | Hepatitis A virus*Leptospira spp.*+Bacillus licheniformis+Bacillus subtilis+Pseudomonas aeruginosa+Clostridium disporicumAspergillus spp.Aflatoxin M1Mycotoxin B1 |
Principal causes and defects in milk and dairy products caused by spoilage microorganisms.
| Kind of defect | Cause | Related microorganism | Reference |
| Pasteurized, sterilized, and UHT milk | |||
| Precipitation when milk added to hot beverage (bitty cream) | Activity of phospholipases and proteinases and fat destabilization | Bacillus spp. | [130] |
| Gelation | Thermoresistant proteinases | Psychrotrophic bacteria (Gram-negative and Gram-positive): Pseudomonas spp. (106–108 cfu mL−1) | [131, 132] |
| Shorter shelf life | Proteolytic and lipolytic activities | Bacillus cereus spp. (106 cfu mL−1) | [130, 133] |
| Undesirable flavor: unclean, fruity, bitter, rancid, yeasty | High concentration of free fatty acids due to activity of thermostable lipases; protein hydrolysis due to activity of heat stabile proteinases | Pseudomonas fragi P. fluorescens | [130] |
| Increase of free fatty acids and casein hydrolyses, destabilizing the casein micelles (acid coagulation of milk) | Proteolytic and lipolytic activities | Bacillus spp. | [133]; [134]; [120] |
| Milk spoiling | Biofilm formation | Consortium of species | [135] |
| Powder milk | |||
| Shorter shelf life, rancidity, and bitterness | Bacterial proteinases and lipases and increase of free fatty acid | Bacillus spp. | [111] |
| Cheese | |||
| Destabilization of the natural plasmin system of milk. Affect the quality of cheese, flavor and texture development, and reduce the yield of the curd | Activity of lipases and proteinases remain in curd that ongoing hydrological changes during ripening; cause spoilage of milk and dairy product. | Psychrotrophic spp. (>103 cfu mL−1) | [114, 136, 137] |
| Change coagulation time and quality of curd (fragile and less compact) | Higher concentration of free amino acids (bacterial proteinases) which stimulates starter culture which growth.Longer coagulation time: higher concentration of free fatty acids (bacterial lipases) which inhibits starter culture growth | ||
| Undesirables flavor: rancid taste in hard cheeses (ripening) | Bacillus spp. (≥106 cfu mL−1) | [133, 138] | |
| Lipases: free fatty acids increase | |||
| Bitterness and off-flavors | [130] | ||
| Fermented milks | |||
| Changes of texture and flavor: more firm gel and higher viscosity, more pronounced syneresis | Psychrotrophic | [139] | |
| Lipolytic changes (free fatty acid): atypical flavor as bitter, rancid, unclean, and fruity | [140] | ||
| Creams and butter | |||
| Reduced shelf lifeRancidity and off-flavorFruity, bitterness, soapy | High concentration of lipases and proteinases in milk (cream)High concentration of free fatty acids (C4-C6; C110-C12) | PsychrotrophicPseudomonas spp.Bacillus spp.Pseudomonas fragiP. fluorescens | [141, 142] |
References
- Dhakane, Rajesh & Gulve, Rekha & Shinde, Anant & Jadhav, Amol & Bhusnar, Satish. (2019). Spoilage and preservation of milk and milk products: A review. 6. 173-179.
- Velázquez-Ordoñez, V., Valladares-Carranza, B., Tenorio-Borroto, E., Talavera-Rojas, M., Varela-Guerrero, J. A. , Acosta-Dibarrat, J., Puigvert, F., Grille, L., Revello, Á. G. , & Pareja, L. (2019). Microbial Contamination in Milk Quality and Health Risk of the Consumers of Raw Milk and Dairy Products. In G. Mózsik, & M. Figler (Eds.), Nutrition in Health and Disease – Our Challenges Now and Forthcoming Time. IntechOpen. https://doi.org/10.5772/intechopen.86182
- https://www.jetir.org/papers/JETIR1906Y95.pdf
- https://egyankosh.ac.in/bitstream/123456789/65956/1/Unit%2015.pdf
- https://basu.org.in/wp-content/uploads/2021/01/9th-pptSources-of-microbial-contamination-of-milk-and-milk-products.pdf
- http://ecoursesonline.iasri.res.in/mod/resource/view.php?id=4986
- https://prezi.com/mvfg7cbbxkem/contaminationpreservation-and-spoilage-of-milk-productsche/
- https://www.slideshare.net/DrMadhuriKaushishLil/contamination-spoilage-and-preservation-of-milk-and-milk-products
- https://www.slideshare.net/AnilShrestha15/contamination-preservation-and-spoilage-of-milk
- https://mugberiagangadharmahavidyalaya.org/images/ques_answer/1587614296Spoilage_of_Milk_and_Milk_Products.pdf
- https://microbenotes.com/spoilage-of-milk-and-milk-products/
- https://www.onlinebiologynotes.com/microbial-spoilage-of-milk-and-milk-products/
