Table of Contents
Streptococcus pneumoniae
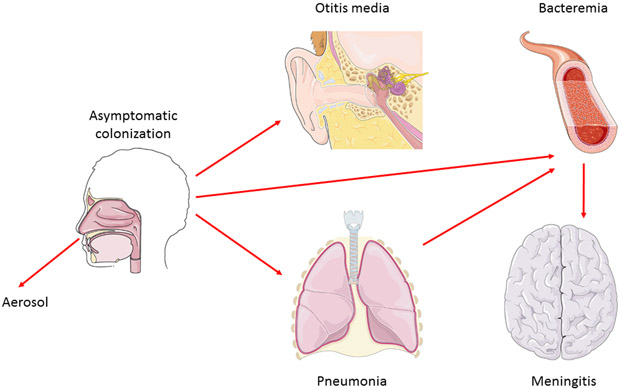
- Streptococcus pneumoniae is a common resident of the upper respiratory tract of humans. The bacterium can cause pneumonia, typically of the lobar variety, paranasal sinusitis, otitis media, and meningitis, which is typically a complication of one of the aforementioned conditions.
- In addition, it can result in osteomyelitis, septic arthritis, endocarditis, peritonitis, cellulitis, and brain abscesses. Currently, Streptococcus pneumoniae is the major cause of invasive bacterial illness in children and the elderly.
- Streptococcus pneumoniae is known as the pneumococcus in medical microbiology because to its appearance and frequent involvement in pneumococcal pneumonia.
- Pneumonia is a lung disease caused by a range of bacteria, such as Streptococcus, Staphylococcus, Pseudomonas, Haemophilus, Chlamydia, and Mycoplasma, as well as many viruses, some fungi, and protozoa. There are two kinds of the disease: bronchial pneumonia and lobar pneumonia.
- The incidence of bronchial pneumonia is highest in newborns, young children, and elderly individuals. Multiple bacteria, including Streptococcus pneumoniae, cause it.
- Alveoli next to the bigger bronchioles of the bronchial tree are affected by bronchial pneumonia. Younger persons are more prone to developing lobar pneumonia. Streptococcus pneumoniae is responsible for the majority (over 80%) of lobar pneumonia cases.
- Lobar pneumonia affects the entirety of a single lobe of the lungs (although multiple lobes may be affected), and the affected area tends to become a solid mass, in contrast to the spongy feel of normal lung tissue.
Characteristics of Streptococcus pneumoniae
- Streptococcus pneumoniae cells are lancet-shaped, Gram-positive cocci (elongated cocci with a slightly pointed outer curvature).
- They are commonly observed as pairs of cocci (diplococci), although they can also appear singly or in short chains.
- When grown on blood agar, they exhibit alpha hemolysis.
- The diameter of each cells ranges between 0.5 and 1.25 micrometres.
- They do not produce spores and are immobile.
- Similar to other streptococci, these bacteria lack catalase and ferment glucose into lactic acid.
- In contrast to other streptococci, they lack a M protein, hydrolyze inulin, and have a unique peptidoglycan and teichoic acid cell wall composition.
- They are part of the typical flora of infections of the upper respiratory tract in humans.
- Typically located in the throat and nasal passages.
- During the winter, they infect the most children.
- Temperatures range from 30 to 35°C.
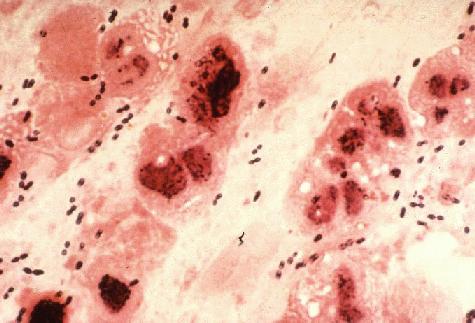
Streptococcus pneumoniae, also known as pneumococcus, is a type of bacteria that typically inhabits the upper respiratory tract of humans, including the nose and throat. It can also be found in other parts of the body, such as the lungs and ears. The bacteria can be transmitted through respiratory droplets, and can cause a variety of infections, including pneumonia, meningitis, and sepsis.
Carriage of pneumococci
Pneumococci are common respiratory tract residents. Depending on the population and environment, the bacteria can be isolated from the nasopharynx of 5–90% of healthy people:
- 5–10% of childless adults are carriers.
- 20–60% of school-aged children may carry the virus
- 50–60% of military personnel on stations may be carriers.
Carriage duration varies and is typically longer for children than for adults. In addition, experts do not comprehend the connection between carriage and the development of natural immunity.
Habitat of Streptococcus pneumoniae
The colon, upper respiratory tract, and skin are among their habitats. But also in our mouth, where Streptococcus mutans resides.
Transmission of Streptococcus pneumoniae
Streptococcus pneumoniae is transmitted through
- Person-to-person transmission of respiratory droplets
- Autoinoculation in carriers of the pathogen in the upper respiratory tract.
The pneumococcal serotypes most usually responsible for generating infection are those most commonly detected in pneumococcal carriers. Carriage may not necessarily result in disease, although it is a critical precursor to pneumococcal infection.
Influencing the spread of an organism within a family or household are the following factors:
Presence of upper respiratory infections or pneumococcal illness, such as pneumonia or otitis media, during the crowded season.
Epidemiology of Streptococcus pneumoniae
- S. pneumoniae is a transient member of the natural flora, occupying the nasopharynx of up to 40 percent of healthy adults and children without causing any harm.
- Children carry this pathogen in the nasopharynx asymptomatically for around four to six weeks, frequently with multiple serotypes. About once every two months, new serotypes are acquired.
- Depending on the region of the world, serotypes 6, 14, 18, 19, and 23 are the most prevalent, accounting for 60-80% of infections. Pneumococcal infection is the leading cause of death among vaccine-preventable bacterial diseases.
- Children between 6 months and 4 years old and those over 60 years old are at the highest risk for pneumococcal infection.
- Before the age of five, virtually every child will suffer pneumococcal otitis media. It is believed that pneumococcus causes 25% of all cases of community-acquired pneumonia (1,000 per 100,000 inhabitants).
- Prior to the year 2000, S. pneumoniae infections caused between 100,000 and 135,000 pneumonia-related hospitalisations, 6 million cases of middle ear infection, and 60,000 cases of invasive illness, including 3,300 cases of meningitis. (In 1997, the CDC reported 60,000 instances of invasive pneumococcal illness, resulting in roughly 6,000 deaths.) Geographic variation in the incidence of sterile-site infections ranges from 21 to 33 incidences per 100,000 people.
- The introduction of combination vaccines is currently altering disease rates. In 2002, the rate of invasive illness in the United States was 13 cases per 100,000 people.
- However, disease epidemics have reemerged in settings such as long-term care institutions, military camps, and day care centres, a phenomenon not seen since the era before antibiotics.
- In the past two decades, the rising occurrence of antibiotic resistance is also cause for concern. Multiple antibiotic resistant strains of S. pneumoniae that arose in the early 1970s in Papua New Guinea and South Africa were supposed to be a fluke; nonetheless, multiple antibiotic resistance is now widespread and has expanded dramatically since 1995.
- Increases in penicillin resistance were followed by increases in resistance to cephalosporins and to multiple drugs. The incidence of penicillin resistance climbed from 0.02 in 1987 to 3% in 1994 to 30% in some U.S. towns and 80% in some regions of other nations in 1998.
- Simultaneously, resistance to various antibiotics has emerged: 26% are resistant to trimethoprim-sulfa, 9% to cefotaxime, 30% to macrolides, and 25% to several medicines.
- However, resistant organisms appear to have emerged in fewer than ten serotypes. The vast majority of resistant strains consist of serotypes 6A, 6B, 9V, 14, 19A, and 23F.
Cultivation of Streptococcus pneumoniae
- Streptococcus pneumoniae is a fussy bacteria that prefers 5% carbon dioxide for optimal growth. Approximately 20% of fresh clinical isolates necessitate anaerobic conditions.
- In all instances, growth requires a source of catalase (such as blood) to neutralise the significant amount of hydrogen peroxide created by the bacteria. At 37 degrees Celsius, the doubling time of the bacterium in complex media including blood is 20 to 30 minutes.
- On agar, pneumococci grow as 1 mm in diameter, sparkling colonies. Types 3 and 37 are mucoid serotypes. At a rate of 1 in 105, pneumococci undergo a genetically regulated phase transition from opaque to transparent colonies.
- The transparent variety is adapted for colonisation of the nasopharynx, whereas the opaque variant is adapted for blood survival. The molecular reason for the difference in colony appearance is unknown, however the expression of surface proteins differs significantly between the two types.
- Streptococcus pneumoniae is an aerotolerant fermenting anaerobe. It is typically grown in medium containing blood. On blood agar, colonies create a zone of alpha (green) hemolysis, which distinguishes S. pneumoniae from the group A (beta hemolytic) streptococcus, but not from commensal alpha hemolytic (viridans) streptococci that occupy the upper respiratory tract.
- To distinguish pneumococcus from Streptococcus viridans, specialised assays, such as inulin fermentation, bile solubility, and optochin (an antibiotic) sensitivity, must be used routinely.
- Streptococcus pneumoniae is an exceptionally delicate bacteria that possesses the enzymatic capacity to break and disintegrate cells. The relevant enzyme is known as an autolysin. This autolysin’s physiological function is to trigger a distinctive autolysis that kills the entire culture when grown to stationary phase.
- Almost all clinical isolates of pneumococci contain this autolysin and undergo lysis between 18 and 24 hours following growth initiation under optimum circumstances.
- Autolysis is associated with colony morphological alterations. Colonies initially have a plateau-like morphology, but when autolysis occurs, the centres begin to collapse.
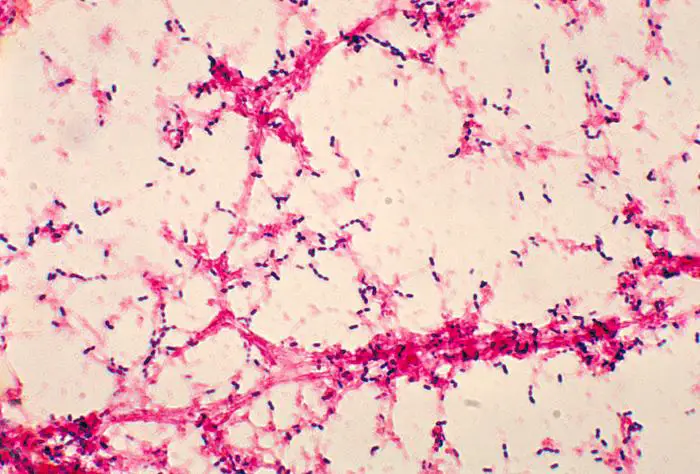
Morphology of Streptococcus pneumoniae
- These microbes are gramme positive.
- A culture’s gramme +ve character may atrophy with time, making it read as gramme -ve.
- They lack the ability to produce spores and move about independently.
- Possible adherence hairs called pili.
- Their dimensions are 0.5 x 1.25 micrometres.
- They usually occur in pairs (diplococci).
- Isolated from bodily fluids, they can also be found individually or in short chains.
- They resemble lancets or bullets in form.
- A capsule protects them from the elements.
- More than 90 different serotypes have been found in capsules.
- Because of their alpha () hemolytic nature, they are deadly.
- More than five hundred distinct surface proteins are present.
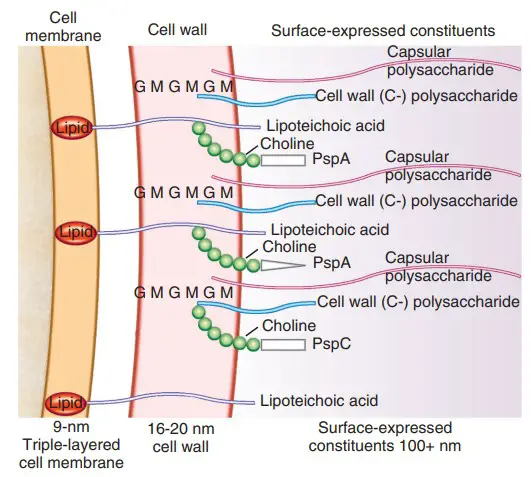
Cell Surface Structure of Streptococcus pneumoniae
1. Capsule
- Pneumococcal cells are entirely enveloped by a polysaccharide capsule. During invasion, the capsule is an important virulence factor.
- By blocking complement C3b from opsonizing bacterial cells, the capsule inhibits phagocytosis. The antigenic serotyping of the organism is based on the identification of 90 different types of pneumococcal capsules.
- Based on formulations of several capsular (polysaccharide) antigens taken from highly prevalent strains, anti-pneumococcal vaccines are created.
2. Cell Wall
- The cell wall of S. pneumoniae is made of peptidoglycan with teichoic acid connected to approximately every third N-acetylmuramic acid and is approximately six layers thick.
- Lipoteichoic acid is chemically identical to teichoic acid, but has a lipid moiety linked to the cell membrane.
- Both teichoic acid and lipoteichoic acid contain phosphorylcholine; two choline residues can be covalently attached to each repeating carbohydrate.
- Choline binds particularly to choline-binding receptors that are present on practically all human cells, making this a crucial component of S. pneumoniae’s biology.
3. Pili
- Recent research has revealed that several strains of S. pneumoniae possess hair-like structures that extend from the surface.
- It has been established that they contribute to colonisation of the upper respiratory tract and boost the immune system’s production of TNF during invasive infection.
4. Surface Proteins
- The pneumococcus is believed to have more than 500 surface proteins based on functional genomic research. Some are physically connected with the cell wall, whereas others are membrane-associated lipoproteins.
- Five penicillin-binding proteins (PBPs), two neuraminidases, and an IgA protease are present in the latter. The family of choline-binding proteins is a distinct group of proteins on the pneumococcal surface (CBPs).
- Twelve CBPs are noncovalently attached to the choline portion of the bacterial cell wall and are utilised to “snap” several functional components onto the bacterial surface.
- CBPs have a C-terminal choline-binding domain, but their N-termini are unique, indicating that their activities are separate. Important virulence factors within the CBP family include PspA (protective antigen), LytA, B, and C (three autolysins), and CbpA. (an adhesin).
Identification of Streptococcus pneumoniae
- Pneumococci can be identified and distinguished from other streptococci based on their sensitivity to bile or optochin, Gram-positive staining, and hemolytic activity.
- On agar containing equine, human, rabbit, and ovine erythrocytes, pneumococci cause alpha hemolysis.
- Under anaerobic conditions, they switch to beta hemolysis, which is produced by an oxygen-sensitive hemolysin.
- Pneumococci typically form a 16-mm zone of inhibition around a 5 mg disc of optochin and are lysed by bile salts (e.g. deoxycholate).
- A few drops of 10% deoxycholate added at 37 degrees Celsius lyses the entire colony within minutes.
- Deoxycholate’s capacity to breakdown the cell wall is contingent on the presence of the autolytic enzyme LytA. Practically all pneumococci clinical isolates include autolysin and undergo deoxycholate lysis.
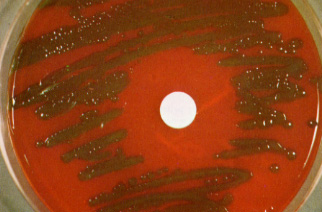
Serotyping of Streptococcus pneumoniae
- The quellung reaction (swelling reaction) is the foundation of serotyping and relies on the swelling of the capsule caused by the binding of homologous antibodies.
- The test comprises of combining a loopful of colony with an equivalent amount of particular antiserum and then observing for capsular enlargement at 1000X magnification.
- Cross-reactivity has been seen between capsular types 2 and 5, 3 and 8, 7 and 18, 13 and 30, as well as E. coli, Klebsiella, H. influenzae Type b, and some viridans streptococci, despite the fact that they are normally very specific.
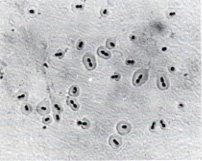
Genetics or Genome of Streptococcus pneumoniae
- S. pneumoniae utilises a natural transformation pathway for genetic exchange. This process is medically significant since it clearly explains the explosive growth of antibiotic resistance in bacteria during the last two decades.
- For instance, penicillin resistance is a result of modified penicillin-binding proteins (PBPs) with reduced affinity for beta lactam antibiotics. Comparing the nucleotide sequences encoding PBPs in S. pneumoniae and S. mitis reveals horizontal gene transfer between these two bacteria.
- S. pneumoniae can be changed in the laboratory utilising genes from related and unrelated bacteria.
- In addition, horizontal exchanges of genetic information may occur in the upper respiratory tract of the host between strains of pneumococci that coexist or struggle for dominance with normal flora.
- Streptococcus pneumoniae can also gain resistance to antibiotics through the timeless process of mutation and natural selection.
- The bacterium has a relatively rapid growth rate and attains high cell densities in an infectious environment. These conditions not only enhance the occurrence of natural transformation, but also the creation of antibiotic-resistant mutants that arise spontaneously.
- The binding, absorption, and assimilation of exogenous DNA during transformation occur as a sequence of regulated actions during a physiologically defined condition called competence.
- Competent bacteria self-aggregate, readily generate protoplasts, are susceptible to autolysis, and have a higher concentration of H+ and Na+, all of which contribute to an increase in glycolysis and ATP reserves.
- During competence, a distinct set of at least 11 proteins is preferentially expressed. Growing bacteria release a 17-amino acid peptide known as competence-stimulating peptide (CSP) early in the competent stage.
- Consistent with a quorum-sensing hypothesis, CSP produces competence when it reaches a critical concentration that relies on the cell density.
Pathogenesis of Streptococcus pneumoniae
Pneumococci cause sickness in people, primates, rabbits, horses, mice, and guinea pigs on their own accord. Approximately 40% of the population is colonised by nasopharyngeal bacteria. Pneumonia and middle ear infection are the most prevalent infections, while meningitis is significantly more unpredictable. The rabbit and mouse have been utilised extensively as disease models, resulting in a decent grasp of numerous pneumococcal virulence determinants.
Colonization
- Pneumococci cling strongly to the nasopharyngeal epithelium by several processes, which, for the majority of individuals, appears to generate type-specific immunity. Some individuals, however, proceed to the lungs or middle ear.
- Passage of pneumococci up the eustachian tube is accompanied by bacterial-driven alterations in the surface receptors of the epithelial cell, specifically those generated by neuraminidase.
- Pneumococcal cell wall components promote inflammation in the middle ear, and pneumolysin causes significant cytotoxicity to the ciliated cells of the cochlea.
- Pneumococci escape the ciliated upper respiratory epithelial cells when reaching the lower respiratory tract via aerosol, unless there is damage to the epithelium. Instead, they move to the alveolus and connect with certain alveolar cells that generate a surfactant containing choline.
- Experimentally, roughly 100,000 bacteria/ml are required to induce an inflammatory response in healthy tissues.
- Nevertheless, if a proinflammatory signal is provided, inflammation can occur with as few as 10 microorganisms. In experimental systems, this signal is a cytokine; in clinical settings, it is an intercurrent viral infection. The inflammatory reaction is capable of causing significant tissue damage.
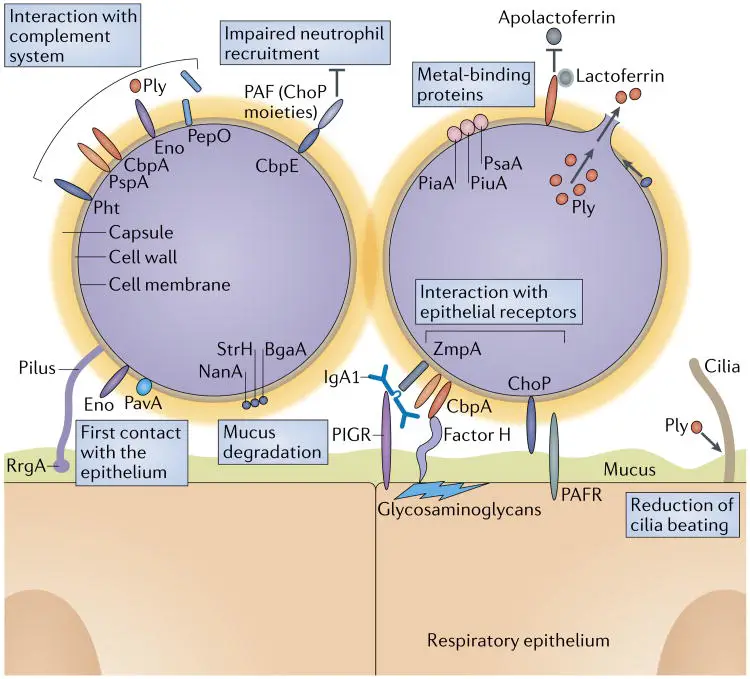
Invasion
- Due to their resistance to the host’s phagocytic response, bacteria are able to penetrate and thrive. The cell wall components directly trigger various inflammatory cascades, including the alternate pathway of complement activation, the coagulation cascade, and the cytokine cascade, causing macrophages and other cells to produce interleukin-1, interleukin-6, and tumour necrosis factor (TNF).
- Additionally, once pneumococci begin to lyse as a result of autolysis or in reaction to host defensins and antimicrobial drugs, they release cell wall components, pneumolysin, and other chemicals that result in increased inflammation and cytotoxic consequences.
- The bacteria’s synthesis of pneumolysin and hydrogen peroxide kills cells and induces the formation of nitric oxide, which may play a crucial role in septic shock.
- The interaction between the bacterial cell wall choline and the human PAF receptor G-protein contributes to an altered state of vascular permeability during invasion. This results in the arrival of an inflammatory exudate in the lung. Initially, a serous exudate develops.
- This is followed by the entry of leukocytes, so transforming a serous exudate into a purulent one. Pneumococcal infection sites are distinguished by the strength of their purulent reaction.
- Pneumococci can occasionally infiltrate endothelial cells directly. Pneumococci bind to activated human cells via choline on the cell wall teichoic acid, which can serve as a direct ligand to the PAF receptor, and the choline-binding protein, CbpA, which binds to a particular carbohydrate on the alveolar cell surface.
- In a receptor-mediated endocytic process, the pneumococcus enters a vacuole when bound to the PAF receptor, and the vacuole spreads across the cell, discharging the bacteria on the ablumenal surface. Pneumococci attach to and pass an endothelial barrier in vitro in around four hours.
- If bacteremia develops, meningitis risk increases. Using the identical pairings of choline to PAF receptor and CbpA to carbohydrate receptor, pneumococci can adhere particularly to brain capillaries.
- Consequently, the bacteria subvert the endocytosis/recycling pathway of the PAF receptor for transmigration of cells. Once in the cerebrospinal fluid, numerous pneumococcal components, particularly cell wall components, provoke an inflammatory response.
Bacterial Determinants of Virulence
Pili
- Attachment of encapsulated pneumococci to epithelial cells in the upper respiratory tract is the initiating step in invasive pneumococcal illness. Pili, which were previously unknown to exist in pneumococci, have recently been found to improve initial bacterial adherence and subsequent invasive disease-causing capabilities.
- These pili-like sticky appendages are encoded by the rlrA islet, which is present in certain clinical isolates but not all. Introduction of the rlrA islet into an encapsulated rlrA-negative isolate enables expression of the pilus, improves adhesion to lung epithelial cells, and confers a competitive advantage during mixed intranasal challenge of mice.
- In addition, pilus-expressing rlrA islet-positive clinical isolates are more virulent than nonpiliated deletion mutants, outcompeting them in mouse models of colonisation, pneumonia, and bacteremia.
- Moreover, piliated pneumococci elicit a greater TNF response during systemic infection than nonpiliated counterparts, indicating that pneumococcal pili not only help to adhesion and pathogenicity but also drive the host’s inflammatory response.
Capsule
- The bacterial capsule interferes with phagocytosis by leukocytes, a feature dependent on its chemical composition. Apparently, resistance to phagocytosis is brought about by interference with binding of complement C3b to the cell surface.
- During invasion of the mucosal surface, encapsulated bacteria are 100,000 times more virulent than unencapsulated ones. The polysaccharide is harmless and noninflammatory, and the capsule does not appear to engage any host defences except for the development of antibody-mediated immunity.
- The pneumococcal capsule is not an antigenic disguise, and it does not limit the activities of underlying components, such as the cell wall and surface proteins, to engage the host defence systems.
- However, C-reactive protein or antibodies to teichoic acid, both of which bind to the cell wall under the capsule, fail to opsonize encapsulated strains.
Cell Wall Components
- The pneumococcal cell wall is a collection of powerful inflammatory triggers. Challenge with cell wall components alone can mimic many of the symptoms of pneumonia, otitis media and meningitis in experimental models.
- The phosphorylcholine adorning the teichoic acid and the lipoteichoic acid is a crucial component facilitating invasion, and works both as an adhesin and as a docking site for the choline-binding proteins (CBPs) (CBPs). Other respiratory infections such as Haemophilus, Pseudomonas, Neisseria and Mycoplasma also carry phosphorylcholine on lipopolysaccharide, proteins or pili, suggesting a common mechanism for invasion of the respiratory system.
- Two host-derived components that identify choline are platelet activating factor (PAF) receptor and the C-reactive protein.
- Since respiratory pathogens may be detected and removed by the C-reactive protein response as part of the innate defences, respiratory pathogens may share this intrusive strategy to subvert the signalling cascade of endogenous PAF.
- The peptidoglycan/teichoic acid combination of the pneumococcus is extremely inflammatory. Smaller components of peptidoglycan increasingly lose particular inflammatory action.
- The cell wall directly activates the alternative pathway of the complement cascade, creating chemotaxins for leukocytes, and the coagulation cascade, which promotes a “procoagulant state” promoting thrombosis.
- In addition, peptidoglycan binds to CD14, a cell surface receptor known to initiate the inflammatory response for endotoxin. This initiates a cytokine cascade culminating in synthesis of interleukin-1, interleukin-6 and tumour necrosis factor from human cells.
Choline Binding Proteins
- The CBP family comprises such key determinants as PspA (protective antigen), LytA, B, and C (three autolysins), and CbpA (an adhesin) (an adhesin).
- The protective antigen (PspA) is a 6 kDa protein with 10 choline-binding repeats. PspA appears to suppress complement-mediated opsonization of pneumococci, and mutants lacking PspA exhibit lower pathogenicity. Antibodies against PspA give passive protection in mice.
- Autolysin LytA is responsible for pneumococcal lysis in stationary phase as well as in the presence of antibiotics. The protein has two functional domains: a C-terminal domain with six choline-binding repeats that attach the protein on the cell wall, and an N-terminal region that gives amidase activity. Autolysin LytB is a glucosaminidase involved in cell separation, and LytC has lysozyme-like activity.
- CbpA is a significant pneumococcal adhesin. It has eight choline-binding repetitions. The adhesin interacts with carbohydrates on the pulmonary epithelial surface carbohydrates. CbpA-deficient mutants are defective in colonisation of the nasopharynx and fail to bind to numerous human cells in vitro. CbpA additionally has been found to bind secretory IgA and complement component C3.
Hemolysins
- Pneumococci, in addition to surface-associated virulence determinants, release exotoxins. Two hemolysins have been described, with pneumolysin being the most potent. Pneumolysin is a 53 kDa protein that can activate complement and cause the lysis of host cells. It is kept intracellularly and released upon pneumococcal lysis.
- Pneumolysin binds to cholesterol and can therefore bind to all cells regardless of their receptors. The protein self-assembles into oligomers to produce transmembrane holes, which ultimately result in cell death.
- Pneumolysin can also promote the generation of inflammatory cytokines, limit the beating of epithelial cell cilia, inhibit lymphocyte proliferation, reduce neutrophil bactericidal activity, and activate complement.
- A second hemolysin activity has been described, although its identity remains unknown. In addition, pneumococci create larger levels of hydrogen peroxide than human leukocytes. This tiny chemical is an effective hemolysin as well.
Pili
- As stated previously, pili contribute to the colonisation of the upper respiratory tract and promote the production of significant quantities of tumour necrosis factor.
Hydrogen peroxide
- Pneumococcal H2O2 damages host cells (e.g., it can induce death in brain cells during meningitis) and has bactericidal effects on competing bacteria such as Staphylococcus aureus.
Neuraminidase and IgA protease
- As with other pathogens, these exoenzymes generated by the bacteria play a presumed role in pathogenicity.
Vaccines
- Given the 90 distinct pneumococcal capsular forms, a vaccination based solely on polysaccharide is not yet viable. Consequently, vaccinations based on a subset of extremely frequent strains have been developed.
- From four in 1945, to fourteen in the 1970s, to the current 23-valent formulation, the number of serotypes in the vaccine has risen (25 mg of each of serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F).
- These serotypes account for 85 to 90 percent of those that cause invasive illness, and the vaccine’s estimated efficiency is sixty percent. However, widespread underuse of the vaccination means that pneumococcus remains the most prevalent infectious agent resulting in hospitalisation across all age groups.
- This is further compounded by the fact that polysaccharides are not immunogenic in children younger than 2 years of age, where a substantial quantity of illness is prevalent. Those at the highest risk of illness, notably those aged 65 or older, are recommended to have a single dose of vaccination for life.
- Since 2000, a heptavalent pneumococcal conjugate vaccination (PCV7) has been recommended in the United States for all children aged 2 to 23 months and for children at risk aged 24 to 59 months.
- The four-dose series is administered at ages 2, 4, 6 and 12-14 months. Protection against invasive pneumococcal infections, particularly septicemia and meningitis, is adequate. Children exposed to a serotype not included in the vaccination, however, are not protected.
- This constraint, along with the tendency of capsular-polysaccharide conjugate vaccines to promote the spread of non-protected serotypes, has prompted research into vaccinations that offer protection across all species.
Treatment
Antibiotics are the first-line treatment for bacterial infections, and pneumococcus is susceptible to their effects. However, the therapy of pneumococcal infections depends on the patient’s strain of illness. Certain strains of S. pneumoniae have developed resistance to particular antibiotics, rendering them ineffective for treatment. S. pneumoniae cases are frequently tested for antibiotic susceptibility during the diagnostic process, and the physician bases treatment decisions on the results.
Vaccination is preferable to illness treatment due to the belief that prevention is preferable to treatment. Two pneumococcal vaccines are available, and both employ the same mechanism: antigen delivery to elicit an immunological response in the recipient. Nonetheless, with the introduction of these vaccines to prevent the spread of S. pneumoniae, it has been noted that certain strains no longer carry the antigens to which the body has been sensitised. Antigens that are more easily conserved between bacterial strains are being sought for use in alternative vaccination methods.
Diagnosis
By cultivating bacterial samples on blood agar, S. pneumoniae is readily identifiable. Due of their hemolytic properties, bacteria will transform blood agar into a dark green colour. However, cultivating bacterial cultures can be time-consuming. Consequently, it is more effective to diagnose an illness utilising contemporary methods.
The first method involves the identification of antigens in urine, which are components of the streptococcus bacterium. Several body fluids include C polysaccharide, a major component of the bacterial cell wall. Immunochromatography is used to detect the presence of certain antigens and is preferable to bacterial culture for diagnosis.
The second method utilises a test that detects S. pneumoniae-specific DNA sequences. The tremendous specificity afforded by using DNA as a target decreases the possibility of error, and these procedures can be executed incredibly rapidly.
FAQ
What is an invasive Streptococcus pneumoniae infection?
Streptococcus pneumoniae is a common bacterium found in the nose and throat of both children and adults. S. pneumoniae can infect the lungs (pneumonia) or the middle ear (otitis media), but it is called “invasive” when it is detected in the blood, spinal fluid (e.g., meningitis), or another place where germs are ordinarily absent.
Who gets S. pneumoniae infections?
Numerous individuals harbour S. pneumoniae without becoming unwell. Children who are not vaccinated, the elderly, and those with compromised immune systems are at the greatest risk for invasive infection; nonetheless, some persons acquire an invasive infection for no obvious cause.
How is S. pneumoniae spread?
The bacteria are passed from person to person by the breathing in droplets produced by an infected person while coughing or sneezing.
What are the symptoms of S. pneumoniae infection?
Signs and symptoms depend on the part of the body affected. Invasive infection often includes fever, chills, and irritability. Headache, stiff neck, confusion, sleepiness, vomiting, and poor feeding can occur with meningitis.
How soon after exposure do symptoms appear?
The incubation period varies. Signs and symptoms of infection can occur within 1-3 days after exposure, but may occur long after exposure.
How is S. pneumoniae diagnosed?
Laboratory testing on blood, pleural fluid, joint fluid, or cerebrospinal fluid (CSF) are needed to confirm the diagnosis of invasive illness.
What is the treatment for S. pneumoniae infection?
The main treatment for invasive S. pneumoniae infection is antibiotics.
What can be done to prevent the spread of S. pneumoniae?
Vaccines can prevent S. pneumoniae infections from becoming invasive. The 13-valent pneumococcal conjugate vaccine (PCV-13) is recommended for all children between the ages of two months and 59 months. The 23-valent pneumococcal polysaccharide vaccine (PPSV-23) is administered to high-risk individuals at least two years old. Both PCV-13 and PPSV-23 are indicated for adults; their use is determined by lifestyle, age, and health status. Consult your physician or local health department to determine if you should receive the vaccine.
Frequent handwashing with soap and water (or, in the absence of soap and water, with alcohol-based hand sanitizers or gels) can help prevent the transmission of numerous viruses and germs. Not sharing food, beverages, or eating utensils with others can also aid in preventing the transmission of infections.
Exposure to a person with a S. pneumoniae infection does not typically necessitate preventative treatment (e.g., antibiotics).
When and for how long is a person able to spread S. pneumoniae?
The infectious period is variable and might extend as long as the pathogen is present in the nose and throat. After taking the appropriate medicines for 1-2 days, a person can no longer transmit S. pneumoniae.
Does past infection with S. pneumoniae make a person immune?
No. Individuals who have previously contracted S. pneumoniae can contract it again.
References
- Ramirez, M., Carriço, J. A., van der Linden, M., & Melo-Cristino, J. (2015). Molecular Epidemiology of Streptococcus pneumoniae. Streptococcus Pneumoniae, 3–19. doi:10.1016/b978-0-12-410530-0.00001-6
- Ramirez, M. (2015). Streptococcus pneumoniae. Molecular Medical Microbiology, 1529–1546. doi:10.1016/b978-0-12-397169-2.00086-x
- Janoff, E. N., & Musher, D. M. (2015). Streptococcus pneumoniae. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 2310–2327.e5. doi:10.1016/b978-1-4557-4801-3.00201-0
- Sá-Leão, R., & Tomasz, A. (2009). Streptococcus Pneumoniae. Encyclopedia of Microbiology, 304–317. doi:10.1016/b978-012373944-5.00207-8
- Weiser, J.N., Ferreira, D.M. & Paton, J.C. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol 16, 355–367 (2018). https://doi.org/10.1038/s41579-018-0001-8
- Dion CF, Ashurst JV. Streptococcus Pneumoniae. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470537/
- Ramirez, M., Carriço, J. A., van der Linden, M., & Melo-Cristino, J. (2015). Molecular Epidemiology of Streptococcus pneumoniae. Streptococcus Pneumoniae, 3–19. doi:10.1016/b978-0-12-410530-0.00001-6
- https://www.frontiersin.org/articles/10.3389/fimmu.2018.01366/full
- https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/pneumococcal-disease
- https://www.vdh.virginia.gov/epidemiology/epidemiology-fact-sheets/streptococcus-pneumoniae-invasive-infection/
- https://www.uptodate.com/contents/invasive-pneumococcal-streptococcus-pneumoniae-infections-and-bacteremia
- https://www.dhs.wisconsin.gov/immunization/pneumo.htm
- https://www.dshs.texas.gov/vaccine-preventable-diseases/streptococcus-pneumoniae-infection-pneumococcal-disease
- https://www.creative-diagnostics.com/tag-streptococcus-pneumoniae-antigens-73.htm
- https://www.nhsinform.scot/illnesses-and-conditions/infections-and-poisoning/pneumococcal-infections
- https://pubmlst.org/organisms/streptococcus-pneumoniae
- https://hhs.iowa.gov/cade/disease-information/streptococcus-pneumoniae
- https://www.cdc.gov/pneumococcal/clinicians/streptococcus-pneumoniae.html#:~:text=Streptococcus%20pneumoniae%20are%20lancet%2Dshaped,the%20majority%20of%20pneumococcal%20infections.
- https://microbenotes.com/habitat-and-morphology-of-streptococcus-pneumoniae/
- https://www.ncbi.nlm.nih.gov/books/NBK470537/
- https://www.news-medical.net/health/Streptococcus-pneumoniae-(pneumococcus)-Overview.aspx
- https://emedicine.medscape.com/article/225811-overview
