Table of Contents
What is Major Histocompatibility Complex II (MHC II molecules)?
- Major Histocompatibility Complex II (MHC II) molecules are a specific class of proteins that belong to the major histocompatibility complex (MHC) family. They are primarily found on certain specialized cells known as antigen-presenting cells, which play a crucial role in initiating immune responses. Examples of antigen-presenting cells include dendritic cells, mononuclear phagocytes, some endothelial cells, thymic epithelial cells, and B cells.
- Unlike MHC Class I molecules that present peptides derived from intracellular proteins, MHC Class II molecules present antigens derived from extracellular proteins. The process of loading a peptide onto a MHC Class II molecule occurs through phagocytosis. Extracellular proteins are internalized into the cell and digested within lysosomes. The resulting epitopic peptide fragments are then loaded onto MHC Class II molecules, which subsequently migrate to the cell surface for presentation.
- In humans, the genes encoding MHC Class II proteins are part of the human leukocyte antigen gene complex (HLA), located on chromosome 6. The specific HLA genes corresponding to MHC Class II are HLA-DP, HLA-DM, HLA-DOA, HLA-DOB, HLA-DQ, and HLA-DR.
- Mutations in the HLA gene complex can lead to a condition called bare lymphocyte syndrome (BLS), which is a type of MHC Class II deficiency. This syndrome is characterized by a compromised immune system due to the impaired expression or function of MHC Class II molecules.
- The MHC, present in the genome of all vertebrates, encompasses a cluster of genes responsible for encoding molecules involved in immune recognition. In humans, the MHC is commonly referred to as the human leukocyte antigen (HLA) system. It is located on chromosome 6 and consists of various genes encoding MHC proteins.
- MHC proteins are crucial for the acquired immune system’s ability to recognize foreign molecules and determine histocompatibility. They are cell surface proteins that bind to peptide antigens and present them on the cell surface, allowing appropriate T-cells to recognize and initiate immune responses against these antigens.
- Within the human MHC, the genes encoding the class I, class II, and class III MHC proteins are particularly significant. MHC Class II proteins, as mentioned earlier, are encoded by genes within the HLA-D region of the genome. These proteins have a limited tissue distribution and are primarily found on antigen-presenting cells such as macrophages, dendritic cells, and B cells. However, the expression of MHC Class II molecules can be induced on other cell types, such as endothelial cells or epithelial cells, by the presence of IFN-γ.
- The antigens presented by MHC Class II molecules are derived from extracellular proteins, enabling the immune system to respond to pathogens and other foreign substances encountered outside the cell. MHC Class II molecules have β1 and β2 subunits, allowing them to be recognized by CD4 co-receptors on T cells. Through their interaction with T helper cells (TCD4+), MHC Class II molecules play a vital role in regulating and directing the immune response to infections, particularly by sampling extracellular peptides from pathogens.
- In summary, MHC Class II molecules are a specific class of proteins found primarily on antigen-presenting cells. They play a crucial role in presenting antigens derived from extracellular proteins to T cells, thereby initiating and regulating immune responses. Mutations in the genes encoding MHC Class II proteins can lead to immune deficiencies, such as bare lymphocyte syndrome.
Cellular Distribution of Major Histocompatibility Complex II (MHC II molecules)
- In contrast to class I MHC molecules, class II molecules are expressed constitutively only by antigen-presenting cells, primarily macrophages, dendritic cells, and B cells; however, thymic epithelial cells and other cell types can be induced to express class II molecules and to function as antigen-presenting cells under certain conditions and in response to stimulation by certain cytokines.
- Among the numerous cell types that express class II MHC molecules, there have been reported to be significant expression discrepancies. In some instances, class II expression is dependent on the stage of cell development.
- Class II molecules, for instance, cannot be found on pre-B cells but are constitutively produced on the membrane of mature B cells.
- Until they are triggered by contact with an antigen, monocytes and macrophages express only modest quantities of class II molecules.
- Due to the fact that each of the classical class II MHC molecules consists of two distinct polypeptide chains that are encoded by different loci, a heterozygous individual expresses not only the parental class II molecules, but also molecules including α and β chains from different chromosomes.
- For instance, an H-2k mouse expresses class II IAk and IEk molecules; an H-2d mice expresses class II IAd and IEd molecules. Four parental class II molecules and four molecules containing one parent’s α chain and the other parent’s β chain are expressed in the F1 offspring produced by mating mice with these two haplotypes.
- Since the human MHC contains three classical class II genes (DP, DQ, and DR), an individual who is heterozygous expresses six parental class II molecules and six molecules containing α and β chain combinations from any parent.
- The existence of numerous -chain genes in mice and humans, and multiple β-chain genes in humans, increases the number of various class II molecules expressed by one individual.
- This diversity likely increases the amount of antigenic peptides that can be presented, which is helpful for the organism.
Structure of Major Histocompatibility Complex II (MHC II molecules)
As stated previously, MHC class II molecules are nearly exclusively located on APCs. The peptides bound by MHC class II are produced from the breakdown of proteins that have entered the cell from the outside via phagocytosis or receptor-mediated endocytosis. Because APCs also catch and digest exhausted host proteins, the vast majority of peptides presented on MHC class II molecules are “self” and do not activate CD4+ T cells because the formation of central tolerance has eliminated these specificities from the Th cell repertoire. When an APC presents a non-self peptide coupled to MHC class II, a Th response is produced.
a. MHC Class II Component Polypeptides
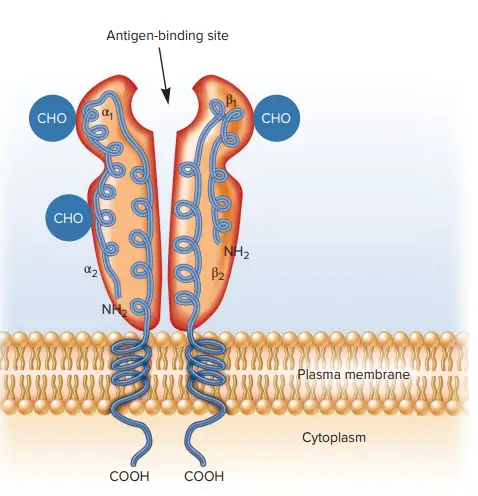
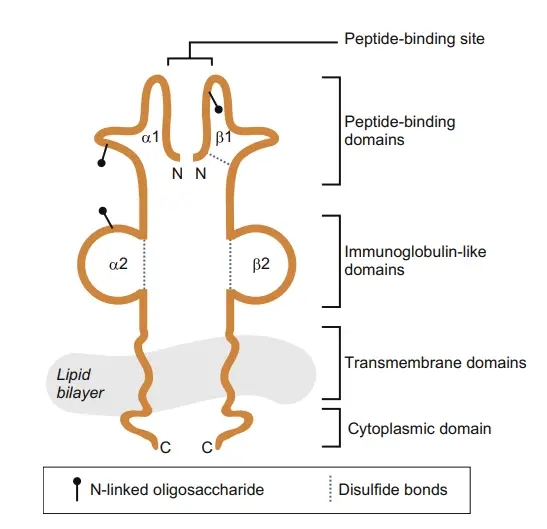
- The α and β chains of MHC class II proteins are glycoproteins of comparable size and structure in both humans and mice (24–32 and 29–31 kDa, respectively).
- Both chains possess an N-terminal extracellular domain, an Ig-like extracellular domain, a hydrophobic transmembrane region, and a short cytoplasmic tail.
- The N-terminal α1 and β1 domains of the α and β chains, respectively, comprise the peptide-binding area.
- The α2 and β2 domains are homologous to the Ig fold but play no role in peptide binding.
b. MHC Class II Peptide-Binding Site
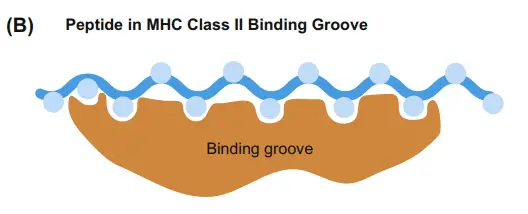
- The peptide-binding groove of MHC class II molecules is structurally similar to that of MHC class I molecules.
- However, the extremities of the MHC class II groove are wide open, allowing for the binding of significantly longer peptides (up to 30 amino acids).
- However, the bulk of peptides discovered in MHC class II grooves range in length from 13 to 18 amino acids.
- The open ends of the MHC class II groove also imply that binding does not rely on conserved anchor residues at the ends of peptides, but is instead mediated by hydrogen bonds between the peptide backbone and the sidechains of specific MHC amino acids.
- Researchers have discovered that antigenic peptides that successfully bind to the floor of the MHC class II groove have a particular conserved secondary structure (resembling a polyproline chain) in the portion of the peptide that aligns with critical acidic MHC residues located in the groove’s centre.
- Due to this conformational requirement, MHC class II proteins typically bind a more limited array of proteins than MHC class I proteins.
Structure of Major Histocompatibility Complex II
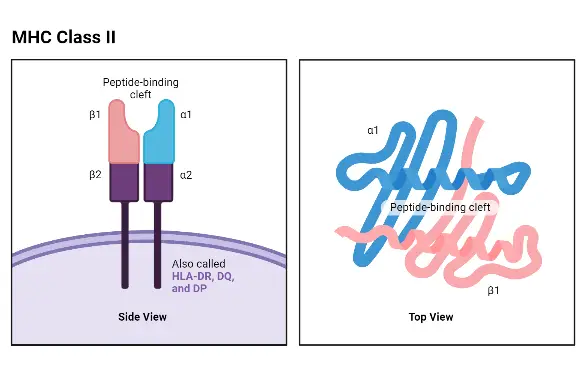
The structure of Major Histocompatibility Complex II (MHC II) molecules is composed of a dimeric arrangement, with a 133 KDa α-chain and a 28 KDa β-chain that associate through non-covalent interactions. Both the α-chain and β-chain consist of two domains each: α1 and α2 for the α-chain, and β1 and β2 for the β-chain.
MHC II molecules are membrane-bound glycoproteins that possess external domains, a transmembrane segment, and a cytoplasmic tail. The peptide-binding cleft, where antigens are bound, is formed between the α-chain and β-chain at the proximal end, creating an open-ended groove. This groove serves as the site for binding antigenic peptides.
Similar to MHC Class I molecules, MHC Class II molecules are heterodimers, but in this case, they consist of two homogenous peptides—an α-chain and a β-chain—both of which are encoded within the MHC genes. The α1, α2, β1, and β2 subdesignations refer to separate domains within the HLA gene, with each domain typically encoded by a different exon. Some genes may have additional domains encoding leader sequences, transmembrane sequences, and so on.
MHC II molecules have extracellular regions, a transmembrane sequence, and a cytoplasmic tail. The α1 and β1 regions of the chains come together to form the membrane-distal peptide-binding domain, while the α2 and β2 regions constitute the remaining extracellular portions of the chains, forming a membrane-proximal immunoglobulin-like domain. The antigen-binding groove, where the antigenic peptide binds, is composed of two α-helix walls and a β-sheet.
One notable structural difference between MHC Class II and Class I molecules is that the antigen-binding groove of MHC Class II molecules is open at both ends, whereas the corresponding groove in Class I molecules is closed at each end. Due to this structural variation, antigens presented by MHC Class II molecules are typically longer, ranging between 15 and 24 amino acid residues in length.
In summary, MHC II molecules consist of a dimeric structure with α-chain and β-chain subunits. They possess distinct domains, including the extracellular regions, transmembrane segment, and cytoplasmic tail. The open-ended groove formed between the α-chain and β-chain serves as the peptide-binding cleft for binding antigenic peptides. This structural arrangement allows MHC II molecules to present longer antigenic peptides compared to MHC Class I molecules.
How are self and non-self peptides deposited in the MHC II binding pocket?
- Class II MHC molecules are distinct from class I MHC molecules in that they bind antigen pieces that originate from outside the cell, undergoing exogenous antigen processing.
- MHC class II molecules can only bind to foreign particles (e.g., bacteria, viruses, poisons) that have been taken up by endo- or phagocytosis. Antigen-presenting cells (APCs) are immune cells that attach non-self antigen to MHC class II molecules. These cells include macrophages, DCs, and B cells.
- Processing of exogenous antigen by macrophages and DCs begins with phagocytosis of the intruder. Instead of phagocytosing, B cells have receptors that bind pathogens or other antigenic substances (e.g., sloughed fragments of microbial cell wall).
- Once antigen has bonded to B-cell receptors, receptor-mediated endocytosis transports the antigen-receptor complex inside the cell.
- In every instance, breakdown in the phagolysosome or autolysosome releases antigenic peptides belonging to the invader. Combining with preexisting class II MHC molecules, these peptides are transported to the cell surface.
- As with class I MHCs, only peptides that fit snugly into the binding pocket of class II MHCs are bound. This peptide is now capable of being identified by CD4+ T-helper cells.
- DCs are especially proficient at presenting foreign peptides to T cells and activating them to become T cells.
- CD4+ T cells do not directly kill target cells, unlike CD8+ T cells. They instead release cytokines to coordinate an integrated innate and adaptive immune response.
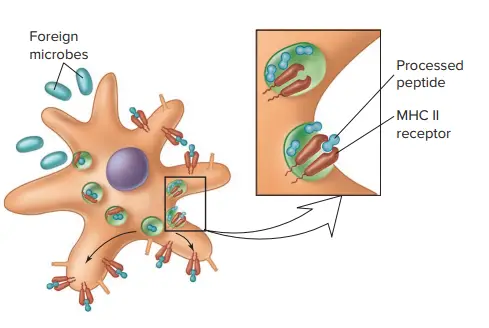
Mechanism of Major Histocompatibility Complex II
- The mechanism of Major Histocompatibility Complex II (MHC II) involves the presentation of antigens derived from exogenous sources to CD4+ T cells, which play a crucial role in immune responses.
- Phagocytes, such as macrophages and immature dendritic cells, uptake exogenous entities through phagocytosis, leading to their internalization into phagosomes. These phagosomes then fuse with lysosomes, forming phagolysosomes. Within the acidic environment of the phagolysosomes, the uptaken proteins are cleaved into numerous peptide fragments by acidic enzymes.
- During the synthesis of MHC Class II molecules, they are transported from the endoplasmic reticulum (ER) through the Golgi apparatus to endosomal compartments. The α and β chains produced are associated with a specific polypeptide called the invariant chain (Ii). The role of the invariant chain is to prevent endogenous peptides from binding to the groove of MHC Class II molecules during their maturation and trafficking.
- In the acidic environment of the endosomal compartments, the invariant chain undergoes degradation, leading to the removal of its fragments, except for a short peptide called the CLIP (class II-associated invariant chain peptide). CLIP blocks the peptide-binding groove of MHC Class II molecules, further preventing the binding of endogenous peptides.
- Antigenic peptides derived from the cleavage of exogenous proteins in the phagolysosomes replace the CLIP peptide in the peptide-binding groove of MHC Class II molecules. This process is facilitated by a specialized protein known as the HLA-DM (DM) molecule, which catalyzes the exchange of CLIP with other peptides.
- Among the available peptides, a specific peptide that exhibits immunodominance, meaning it has a high affinity for MHC Class II molecules and a strong immunological response, is preferentially loaded onto MHC Class II molecules.
- The peptide-loaded MHC Class II molecules are then transported to the cell membrane surface, where they are presented to CD4+ T cells. The peptide:MHC Class II complex is recognized by the cognate T cell receptor (TCR) on helper T cells. The interaction between the peptide:MHC Class II complex and the TCR initiates signaling pathways, leading to the activation of the CD4+ T cell and subsequent immune responses.
- In summary, the mechanism of MHC Class II involves the uptake of exogenous entities by phagocytes, cleavage of the uptaken proteins into peptide fragments, trafficking of MHC Class II molecules with the invariant chain to endosomal compartments, removal of the invariant chain fragments, binding of antigenic peptides to the MHC groove, preferential loading of an immunodominant peptide, transportation of peptide-loaded MHC Class II molecules to the cell membrane surface, and recognition of the peptide:MHC Class II complex by CD4+ T cells through their TCRs.
Functions of Major Histocompatibility Complex II (MHC II molecules)
- MHC II is crucial for the induction and regulation of adaptive immunity.
- It plays a role in selecting the mature CD4+ T cell repertoire in the thymus.
- MHC II activates CD4+ T cells in the periphery.
- The engagement between TCR and peptide-MHC II complex is important for adaptive immunity.
- Secure attachment of the peptide to MHC II ensures stable binding.
- Stable peptide binding enhances T cell recognition of the antigen.
- MHC II facilitates T cell recruitment.
- MHC II plays a critical role in initiating the antigen-specific immune response.
- MHC II molecules sample and present antigens from exogenous sources.
- Antigen-presenting cells (APCs) capture and break down antigens into peptide fragments.
- Peptide fragments bind to MHC II molecules within APCs.
- Loaded MHC II molecules are transported to the cell surface for antigen presentation.
- TCR interaction with peptide-MHC II complex triggers T cell activation.
- T cell activation leads to proliferation and differentiation of CD4+ T cells.
- Effector T cells are generated to combat the pathogen or provide help to other immune cells.
- MHC II contributes to T cell recruitment in lymphoid tissues.
- T cell recruitment is targeted to specific antigens presented by MHC II.
- Secure attachment of peptide to MHC II enhances T cell recognition and immune response effectiveness.
FAQ
What are MHC II molecules?
MHC II molecules are a class of major histocompatibility complex molecules that are primarily found on antigen-presenting cells such as dendritic cells, macrophages, and B cells. They play a crucial role in presenting antigens derived from exogenous sources to CD4+ T cells.
What is the function of MHC II molecules?
The main function of MHC II molecules is to bind to peptide antigens derived from extracellular proteins and present them on the cell surface for recognition by CD4+ T cells. This interaction is crucial for the initiation and regulation of adaptive immune responses.
How do MHC II molecules load antigens?
MHC II molecules load antigens through a process called phagocytosis. Antigens are taken up by antigen-presenting cells, processed into peptide fragments in the phagolysosomes, and then loaded onto MHC II molecules within endosomal compartments.
What is the structure of MHC II molecules?
MHC II molecules are composed of two subunits: an α-chain and a β-chain. Each chain consists of two domains (α1, α2, β1, and β2) and has extracellular regions, a transmembrane segment, and a cytoplasmic tail. The peptide-binding groove is formed between the α-chain and β-chain.
Are MHC II molecules encoded by specific genes?
Yes, in humans, the MHC II protein complex is encoded by the human leukocyte antigen gene complex (HLA). The genes corresponding to MHC II molecules are HLA-DP, HLA-DM, HLA-DOA, HLA-DOB, HLA-DQ, and HLA-DR.
What happens if there are mutations in the MHC II genes?
Mutations in the HLA gene complex can lead to a condition called bare lymphocyte syndrome (BLS), which is a type of MHC II deficiency. This condition impairs the ability of cells to properly present antigens to CD4+ T cells, resulting in compromised immune responses.
Can MHC II molecules present endogenous antigens?
No, MHC II molecules primarily present antigens derived from extracellular proteins, not endogenous antigens. The peptide fragments loaded onto MHC II molecules are generated from proteins that have been phagocytosed and processed within antigen-presenting cells.
How do MHC II molecules interact with CD4+ T cells?
The peptide:MHC II complex on the surface of antigen-presenting cells is recognized by the T cell receptor (TCR) on CD4+ T cells. This interaction triggers signaling pathways in the T cell, leading to T cell activation, cytokine secretion, and coordination of immune responses.
What is the role of MHC II in immune responses?
MHC II molecules are critical for initiating antigen-specific immune responses. By presenting antigens to CD4+ T cells, MHC II molecules play a crucial role in activating T cell-mediated immune responses and coordinating the overall immune response to infections and diseases.
Can MHC II molecules be found on all cell types?
MHC II molecules are primarily found on professional antigen-presenting cells, such as dendritic cells, macrophages, and B cells. However, under certain conditions, other cell types, such as endothelial cells or epithelial cells, can also express MHC II molecules when induced by specific immune signals like IFN-γ.
References
- Kindt, T., Goldsby, R., Osborne, B., Kuby, J. and Kuby, J. (2007). Kuby immunology. New York: W.H. Freeman.
- Natarajan K, Li H, Mariuzza RA, Margulies DH. MHC class I molecules, structure and function. Rev Immunogenet. 1999;1(1):32-46. PMID: 11256571.
- Li XC, Raghavan M. Structure and function of major histocompatibility complex class I antigens. Curr Opin Organ Transplant. 2010 Aug;15(4):499-504. doi: 10.1097/MOT.0b013e32833bfb33. PMID: 20613521; PMCID: PMC3711407.
- Natarajan, K & Li, Hongmin & Mariuzza, RA & Margulies, David. (1999). MHC class I molecules, structure and function. Reviews in immunogenetics. 1. 32-46.
- Wieczorek, M., Abualrous, E. T., Sticht, J., Álvaro-Benito, M., Stolzenberg, S., Noé, F., & Freund, C. (2017). Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Frontiers in Immunology, 8. doi:10.3389/fimmu.2017.00292
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. The major histocompatibility complex and its functions. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27156/
- Hohl, T. M. (2015). Cell-Mediated Defense against Infection. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 50–69.e6. doi:10.1016/b978-1-4557-4801-3.00006-0
- Mak, T. W., & Saunders, M. E. (2006). MHC: The Major Histocompatibility Complex. The Immune Response, 247–277. doi:10.1016/b978-012088451-3.50012-0
- The Major Histocompatibility Complex. (2014). Primer to the Immune Response, 143–159. doi:10.1016/b978-0-12-385245-8.00006-6
- Rammensee, H.G. (1993). Structure and Function of MHC Class I Molecules. In: Eibl, M.M., Huber, C., Peter, H.H., Wahn, U. (eds) Symposium in Immunology I and II. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-78087-5_9
- https://www2.nau.edu/~fpm/immunology/documents/Ch-08.pdf
- https://www.ndvsu.org/images/StudyMaterials/Micro/MHC-Antigens.pdf
- https://pdb101.rcsb.org/motm/62
- https://www.jrc.ac.in/working_folder/DOWNLOAD-D-11-53-5F0F117D553FB.pdf
- https://www.microbiologybook.org/bowers/mhc.htm
- https://www.azolifesciences.com/article/What-is-the-Function-of-MHC-Molecules.aspx
- https://www.onlinebiologynotes.com/major-histocompatibility-complex-mhc-structure-types-and-functions/
- https://immunobites.com/2018/07/23/what-is-mhc-and-why-does-it-matter/
- https://microbenotes.com/major-histocompatibility-complex-i-structure-mechanism-and-functions/
