Table of Contents
- Hugh and Leifson published the details of the oxidative-fermentative (OF) test in 1953.
- “had discovered that some bacteria made acid from carbohydrates solely under aerobic settings and others created acid both under aerobic and anaerobic conditions,” the authors write of previous microbiological research.
- Fermentation was once characterised as the process by which acid was produced through the metabolism of carbohydrates in both aerobic and anaerobic metabolism.
- The term “oxidative” was coined by Hugh and Leifson to describe the process by which acid is produced from carbohydrates in the presence of oxygen.
- It was discovered that aerobic carbohydrate use by bacteria results in less acid production than does fermentative metabolism.
- Hugh and Leifson came up with OF medium to separate the bacterial populations. Glycogen was the primary source of carbohydrate in the first OF medium.
- To make this medium, we reduced the amount of peptone and increased the amount of glucose over what is seen in medium used to detect fermentation.
- Therefore, the alkaline byproduct of peptone metabolism was reduced and acid generation was increased, even during oxidative metabolism, in this medium.
- For the first time, scientists were able to clearly differentiate between gram-negative bacteria that oxidise glucose and those that ferment it using this medium.
- These features eventually proved crucial for the classification of gram-negative bacteria.
- Taxonomists “gave little attention to the type of carbohydrate breakdown” prior to the study of Hugh and Liefson.
- According to Cowan and Steele, “this test is one of the most significant in the identification of aerobic bacteria,” which they wrote about in their 1965 manual, Manual of the Identification of Medical Bacteria.
- Most bacterial genera are made up of bacteria that oxidise glucose or ferment it, therefore when a genus has species that attack glucose in both of these ways, it may be time to rethink its taxonomy.
Purpose of OF Test
- The oxidative-fermentative test is used to find out if gram-negative bacteria use fermentation or oxidation to break down carbohydrates or if they are nonsacchrolytic and can’t use the carbohydrates in the medium.
Principle of OF Test
- The oxidative-fermentative test distinguishes between the fermentation and aerobic respiration pathways for glucose metabolism in certain gram-negative rods (oxidatively).
- The different types of anaerobic fermentation result in different mixtures of acids formed from pyruvate.
- The bromthymol blue indicator in OF medium changes colour from green to yellow depending on the presence or absence of oxygen due to the high concentration of acid produced during fermentation.
- Aerobic respiration is used by nonfermenting gram-negative bacteria, leading to the production of weak acids during the Krebs cycle and the work of Entner Doudoroff (glycolysis).
- These weak acids are produced to a level detectable by the bromthymol blue indicator due to the elevated content of glucose in the medium.
- This medium’s low content of peptones helps with the detection of weak acids.
- The metabolism of amino acids produces amines, which have a neutralising effect.
- The addition of a dipotassium phosphate buffer helps with acid detection. This reaction is indicative of the oxidative nature of the bacteria growing in OF medium.
Hugh and Leifson’s OF basal medium
Composition of Hugh and Leifson’s OF basal medium
| Ingredients | g/l |
| Peptone (tryptone) | 2.0 g |
| Sodium chloride | 5.0 g |
| Glucose (or other carbohydrate) | 10.0 g |
| Bromthymol blue | 0.03 g |
| Agar | 3.0 g |
| Dipotassium phosphate | 0.30 g |
Preparation
- Add distilled water to reach 1 litre. Before autoclaving, the pH should be changed to 7.1.
- After the medium has been autoclaved at 121°C for 15 minutes, a 10% solution of carbohydrate that has been filtered and sterilised is added aseptically to bring the final concentration to 1%.
- The carbohydrate-containing sterile medium is put into sterile test tubes aseptically and cooled so that the tubes don’t tilt.
- Before sterilisation, some methods call for 10 g/liter of carbohydrate to be added to the medium.
- The medium is then dissolved by boiling it on a hot plate or steaming it for 20 minutes before putting small amounts of it into test tubes.
- So that the carbohydrate doesn’t break down, the tubed medium is then steamed for 20 minutes instead of being put in an autoclave.
- Companies that sell biological supplies sell OF basal medium that has already been mixed.
- The source of carbs is not included and needs to be added, as explained above.
Procedure of OF (Oxidation-Fermentation) Test
Method 1: Oxidative-fermentative test using OF media with glucose
A. Inoculation of media
- The test organism is put into two tubes of oxidative-fermentative medium by sticking a needle “half way to the bottom,” or 14 inch from the bottom.
- Put 1 cm of mineral oil on top of one of the two tubes. This layer stops oxygen from getting into the medium, so there is no oxygen in the tube.
B. Incubation conditions
- Most gram-negative rods should be left to grow at 35°C for 48 hours.
- If the bacteria are slow to grow, it may take 3 to 4 days to see results.
Method 2: The oxidative-fermentative test using carbohydrates other than glucose
- Nonfermenting gram-negative rods that give an oxidative result in an OF glucose test can be tested further to see if they can oxidatively metabolise other carbohydrates.
- Maltose, lactose, mannitol, or sucrose are used instead of glucose in the medium, and only one tube is used for each type of carbohydrate.
- Many of these nonfermenters grow slowly, so you should use a lot of inoculum.
- Since the test is based on aerobic respiration, there is no need for mineral oil or agar layer.
- After 24 hours, an acid production and a change in pH at the top of the tube show that the test was successful.
- Some slow-growing nonfermenters may need a few days to make enough acid for the bromthymol blue to find them.
Interpretation and results of OF (Oxidation-Fermentation) Test
| Open (Aerobic) Tube | Covered (Anaerobic) Tube | Metabolism |
| Acid (yellow) | Alkaline (green) | Oxidative |
| Acid (yellow) | Acid (yellow) | Fermentative |
| Alkaline (green) | Alkaline (green) | Non-saccharolytic (glucose not metabolized) |
Fermentative results
Bacteria that can ferment glucose produce acid in both the open (aerobic) tube and the oil-covered (anaerobic) tube, which shows that fermentation is happening. The acid that is made (pH 6.0) turns bromthymol blue from green to yellow, which is a sign of the pH. The semisolid texture of the medium also makes it possible to find movement. Away from the stab line, you can see hazy growth.

Oxidative results
When glucose is broken down by bacteria that don’t ferment, the process is called oxidative metabolism. A small amount of acid being made in the open tube shows that this is what happened. The acid that is made (pH 6.0) turns bromthymol blue from green to yellow, which is a sign of the pH. After 24 hours, a change in pH can be seen at the open tube’s surface, where growth can be seen when oxygen is present. With more than 48 hours of incubation, the lower concentration of agar in the medium makes it possible for the weak acid to spread through the whole tube. The oil-covered tube doesn’t change colour or do anything else.
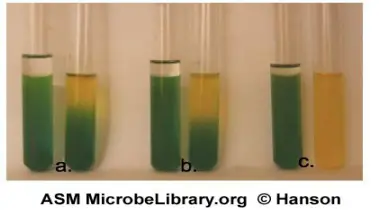
Negative results
The OF result is negative for bacteria that don’t break down sugars. If the oil-covered tube stays the same colour, the result is negative. If the pH goes up to 7.6, the colour of the bromthymol blue at the top of the open tube changes from green to blue. Bacteria that break down the protein in the medium, called peptone, make amines. This is why the pH is going up. Other bacteria give a negative result, which is shown by the medium not growing or changing colour.

Precautions
- It is important to note if the OF test came back negative (N), fermentative (F), or oxidative (O) (-). There is no plus sign (+) for a good outcome. A negative result means that no carbohydrate in the media was metabolised by fermentation or oxidation.
- Avoid squeezing the screw cap tubes too tightly if you use them.
- Because OF medium can change colour when exposed to incubation temperatures, it is important to include incubated and nonincubated, uninoculated controls in your experiments.
Limitations of Oxidation-Fermentation (OF) Test
- Because it is a multipurpose medium, OF media may not be suitable for the development of picky organisms.
- Results from experiments with organisms with a slow growth rate might not be seen for several days.
Quality Control
- Glucose Fermenter: Escherichia coli (Acid production (Yellow), positive reaction in both Aerobic fermentation and Anaerobic fermentation)
- Glucose oxidizer: Pseudomonas aeruginosa (Acid production (Yellow ), positive reaction in Aerobic fermentation and unchanged (green) or alkaline (blue), negative reaction in Anaerobic fermentation)
- Nonsaccharolytic: Moraxella species
References
- Baron, E., and S. Finegold. 1990. Bailey and Scott’s diagnostic microbiology, 8th ed. The Mosby Company, St. Louis, MO.
- Blair, J., E. Lennette, and J. Truant. 1970. Manual of clinical microbiology. American Society for Microbiology, Washington, DC.
- Cowan, S., and K. Steele. 1965. Manual for the identification of medical bacteria. Cambridge University Press, New York, NY.
- Hugh, R., and E. Leifson. 1953. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram-negative rods. J. Bacteriol. 66:24–26.
- Leboffe, M., and B. Pierce. 2006. Microbiology laboratory theory and application, 2nd ed. Morton Publishing Company, Englewood, CO.
- MacFaddin, J. F. 2000. Biochemical tests for identification of medical bacteria, 3rd ed. Lippincott, Williams, and Wilkins, Philadelphia, PA.
- https://microbiologyinfo.com/of-test/
