Table of Contents
What is Immunoelectrophoresis?
- Immunoelectrophoresis is a biochemical technique used for the separation and characterization of proteins based on their electrophoretic mobility and reaction with antibodies. It combines the principles of electrophoresis and immunodiffusion to analyze complex protein mixtures and detect specific antigens.
- The process involves the migration of antigens through an agarose gel under the influence of an electric field. The antigens are first separated into individual components according to their charge and size during electrophoresis. Then, specific antibodies (also known as antisera) are added to troughs parallel to the electrophoretic migration.
- As the antibodies diffuse towards the migrating antigens, antigen-antibody interactions occur, leading to the formation of precipitin lines or arcs. The presence and characteristics of these precipitin lines, such as their intensity, shape, and position, provide information about the antigens present in the sample.
- Immunoelectrophoresis is a versatile technique used in various fields, including clinical diagnostics, research, and immunology. It allows for the identification and quantification of proteins in biological samples, detection of abnormal proteins (e.g., myeloma proteins), analysis of immune-related diseases, and monitoring therapeutic responses.
- Overall, immunoelectrophoresis is a valuable tool for studying the interactions between antigens and antibodies, providing valuable insights into protein composition and immune system-related conditions.
Types of Immunoelectrophoresis
There are 4 types of Immunoelectrophoresis;
- Classical immunoelectrophoresis
- Counter Current Immunoelectrophoresis
- Laurell’s rocket electrophoresis
- Two Dimensional Immunoelectrophoresis
1. Classical immunoelectrophoresis
- Classical immunoelectrophoresis is a technique developed by Grabar and Williams in 1953, which revolutionized protein identification and immunology. In this technique, serum proteins are separated in an agar gel, and antibodies are allowed to diffuse in the gel, leading to the formation of precipitation arcs.
- The procedure begins with a plain agar layer on a microscopic slide. Wells are created in the agar for adding the antigen mixture at the center of the slide, and a trough is cut parallel to the direction of antigen migration for the addition of the antibody. The slide is then placed in an electrophoretic apparatus, and a direct electric current is applied for about 1-2 hours.
- During electrophoresis, the charged antigens present in the mixture separate based on their size and charge. The electric field causes them to migrate at different rates, leading to their spatial separation within the gel.
- After electrophoresis, the specific antisera are added to a trough in the agar, and the slide is incubated overnight in a humid chamber at room temperature. During this incubation period, the antigens and antibodies diffuse into the gel, and where their equivalent concentrations meet, immunoprecipitation occurs.
- The immunoprecipitation appears as an arc due to the radial diffusion of the antigen and the lateral diffusion of the antibody from the trough. The shape and pattern of the immunoprecipitation arc depend on the concentration of the antigen.
- By analyzing the immunoprecipitation arcs, important proteins present in the antigen mixture can be identified. Classical immunoelectrophoresis provides a powerful combination of immunoprecipitation specificity and electrophoretic separation, enabling the separation and characterization of proteins in complex mixtures.
- This technique played a pivotal role in redefining the field of protein identification and immunology by demonstrating the presence of various globulin fractions and important proteins like α1-antitrypsin, α2-macroglobulin, transferrin, C3, and immunoglobulins (IgG, IgA, and IgM). Classical immunoelectrophoresis continues to be a valuable tool in protein analysis and immunological research.
Advantages
- Separation of complex mixtures: Classical immunoelectrophoresis allows for the separation and analysis of complex mixtures of antigens.
- Identification of individual components: The technique enables the identification and analysis of individual components within a complex mixture in a single experiment.
- Specificity: Immunoprecipitation reactions in classical immunoelectrophoresis are specific to the target antigens and their corresponding antibodies, ensuring accurate identification and characterization.
- Simple procedure: The technique involves a relatively simple and straightforward procedure using agarose gel and basic laboratory instruments.
- Visual results: Immunoprecipitation arcs formed during the procedure provide visual results that can be easily interpreted.
- Information-rich arcs: The shape, position, and intensity of the immunoprecipitation arcs offer valuable information about the presence and concentration of specific antigens.
In summary, classical immunoelectrophoresis offers the advantages of separating complex mixtures, identifying individual components, specificity in immunoprecipitation reactions, simplicity in procedure, and providing visual and informative results.
2. Counter Current Immunoelectrophoresis
Counter current immunoelectrophoresis, also known as crossover immunoelectrophoresis, utilizes the different charges of gamma globulin (IgG) and antigens to facilitate immunoprecipitation. Here are some key points about this technique:
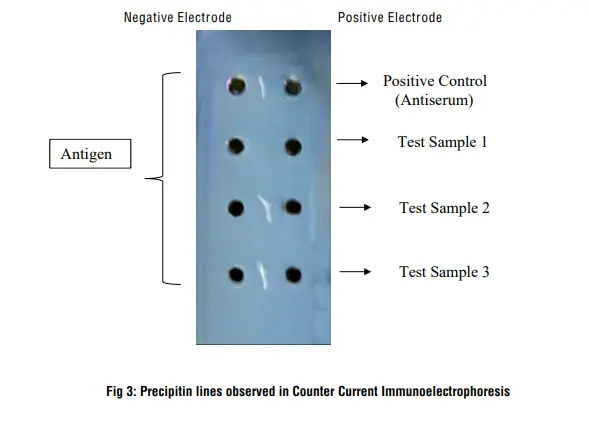
- Charge difference: In counter current immunoelectrophoresis, the gamma globulin (IgG) is positively charged (cation) at the high pH of around 8.0. However, most other proteins in the mixture possess a net negative charge (anion) under such alkaline conditions.
- Loading and electric field: In this technique, the IgG is loaded towards the anode (+ve electrode), while the antigen mixture is loaded towards the cathode (-ve electrode). An electric field is applied to induce movement.
- Migration and immunoprecipitation: As the electric current is passed, the IgG and antigen mixture move towards each other due to their opposite charges. Immunoprecipitation occurs at the zone of equivalence, where the concentration of IgG and antigens is equivalent.
- Formation of immunoprecipitation: At the zone of equivalence, the positively charged IgG and negatively charged antigens interact, leading to the formation of immunoprecipitation. This indicates the presence of specific antigen-antibody interactions.
Counter current immunoelectrophoresis exploits the charge differences between IgG and antigens to facilitate their movement towards each other and promote immunoprecipitation. This technique offers a unique approach to identify and analyze antigen-antibody interactions in a controlled electric field.
Advantage
The advantage of counter current immunoelectrophoresis, also known as crossover immunoelectrophoresis, is its faster speed compared to traditional immunodiffusion techniques. Here are the key points about this advantage:
- Accelerated immunoprecipitation: In counter current immunoelectrophoresis, the antigen and antibody move towards each other under the influence of the electric field. This directed movement accelerates the formation of immunoprecipitation.
- Reduced diffusion time: Unlike traditional immunodiffusion techniques, where the diffusion of antigens and antibodies is solely based on concentration gradients, counter current immunoelectrophoresis expedites the movement of these components. As a result, the overall process is much faster, typically taking around 15-20 minutes to observe immunoprecipitation.
- Rapid results: The accelerated immunoprecipitation in counter current immunoelectrophoresis allows for quicker detection and analysis of antigen-antibody interactions. This rapidity is especially advantageous in situations where timely results are crucial, such as in diagnostic testing or research experiments.
By leveraging the directed movement of antigens and antibodies under the influence of the electric field, counter current immunoelectrophoresis significantly reduces the diffusion time and offers a faster alternative to traditional immunodiffusion techniques. This advantage enables more efficient and timely analysis of antigen-antibody interactions.
Applications
Counter-current immunoelectrophoresis is a valuable technique with various applications in the detection of microbial antigens and other biomolecules in different body fluids and extracts. Here are some key applications of this technique:
- Microbial antigen detection: Counter-current immunoelectrophoresis is commonly used for the identification and detection of microbial antigens in body fluids such as blood, sputum, urine, and tissue or cell extracts. It can be employed in the diagnosis of infectious diseases caused by bacteria, viruses, fungi, and parasites.
- Serological testing: This technique is utilized in serological testing to detect specific antibodies present in patient samples. By using specific antigens, counter-current immunoelectrophoresis can identify the presence of antibodies produced as a result of an immune response to various pathogens.
- Vaccine development: Counter-current immunoelectrophoresis plays a role in vaccine development by assessing the immune response to candidate vaccines. It can help determine the presence and levels of specific antibodies generated by vaccinated individuals, providing insights into the effectiveness of the vaccine.
- Research studies: Counter-current immunoelectrophoresis is utilized in research studies to investigate antigen-antibody interactions, analyze complex mixtures of proteins, and characterize specific biomolecules. It allows researchers to study immune responses, protein-protein interactions, and antigenic profiles in various biological samples.
- Veterinary diagnostics: Counter-current immunoelectrophoresis finds applications in veterinary diagnostics for the detection of microbial antigens and antibodies in animals. It aids in the diagnosis of infectious diseases in livestock, pets, and wildlife, contributing to disease surveillance and control efforts.
The versatility of counter-current immunoelectrophoresis makes it a valuable tool in various fields, including clinical diagnostics, vaccine development, research studies, and veterinary medicine. Its ability to detect microbial antigens and antibodies in different body fluids enables its wide-ranging applications in the detection and characterization of infectious diseases.
3. Laurell’s rocket electrophoresis
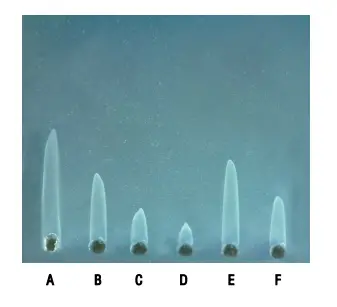
- Laurell’s rocket electrophoresis, also known as electroimmunoassay, is a quantitative one-dimensional immunoelectrophoresis technique developed by Laurell in 1966. This method enables the measurement and quantification of antigen concentrations in samples. The primary application of Laurell’s rocket electrophoresis is in the field of immunology and biomedical research.
- The procedure begins by preparing a gel matrix, typically agar, on a slide. Antibodies specific to the target antigen are added and immobilized within the gel. Wells are then created in the gel, into which the samples containing the antigens of interest are introduced. The slide is placed in an electrophoretic apparatus, and an electric field is applied to facilitate the migration of the antigens.
- Under the influence of the electric field, the antigens move towards the anode. As they migrate, immunoprecipitation occurs when the antigens encounter their corresponding immobilized antibodies in the gel. The immunoprecipitation takes on the shape of a rocket, with most of the antibody-antigen complexes concentrated at the tip of the rocket. However, a small amount of antigen diffuses sideways, resulting in fine precipitation lines along the sides of the rocket.
- Over a period of approximately 1 to 10 hours, the precipitation arc stabilizes and becomes stationary. The height of the rocket formed is directly proportional to the concentration of the antigen in the sample. By plotting the rocket heights on one axis and the known antigen concentrations on the other, a calibration curve can be generated. The calibration curve serves as a reference to determine the concentration of unknown antigen samples based on their respective rocket heights.
- Laurell’s rocket electrophoresis provides a quantitative means of assessing antigen concentrations in samples. Its ability to generate calibration curves allows for accurate determination of unknown antigen concentrations. This technique has found applications in various fields, including clinical diagnostics, immunology research, and pharmaceutical development.
Advantages
Laurell’s rocket electrophoresis offers several advantages that make it a valuable technique for protein analysis and quantification. Some of these advantages include:
- Simplicity and Speed: Laurell’s rocket electrophoresis is a relatively straightforward technique that can be performed with ease. The method does not require elaborate equipment or specialized training, making it accessible to researchers with varying levels of expertise. Additionally, the procedure itself is quick, allowing for rapid analysis of protein samples and efficient processing of multiple samples in a short period.
- Reproducibility: Rocket electrophoresis has demonstrated high reproducibility, ensuring consistent and reliable results. This characteristic is essential in scientific research and clinical applications, where the accuracy and precision of protein quantification are paramount. The method’s reproducibility contributes to the overall reliability of the technique and enhances confidence in the obtained data.
- Specific Protein Quantification: Laurell’s rocket electrophoresis excels in quantifying the concentration of a specific protein within a complex mixture. By using specific antibodies against the target protein, the technique allows for precise determination of the protein’s concentration, even in the presence of numerous other proteins. This specificity is particularly advantageous when studying protein mixtures derived from biological samples such as serum, tissue extracts, urine, cerebrospinal fluid, and more.
- Analysis of Multiple Samples: One of the notable advantages of rocket electrophoresis is the ability to analyze several unknown samples simultaneously on a single plate. This feature streamlines the experimental process, saves time, and reduces the required resources. Researchers can compare and assess multiple samples side by side, providing a comprehensive view of protein concentrations across various conditions or sample types.
Laurell’s rocket electrophoresis has proven to be a valuable tool in protein analysis, offering simplicity, speed, reproducibility, and the ability to quantify specific proteins within complex mixtures. By harnessing these advantages, researchers can gain insights into protein composition, study protein changes in different conditions, and contribute to advancements in various scientific and clinical fields. For further information on the application of this technique, the paper titled “Transferrin changes in haemodialysed patients” by Formanowicz and Formanowicz, 2011, can be referenced.
4. Two Dimensional Immunoelectrophoresis
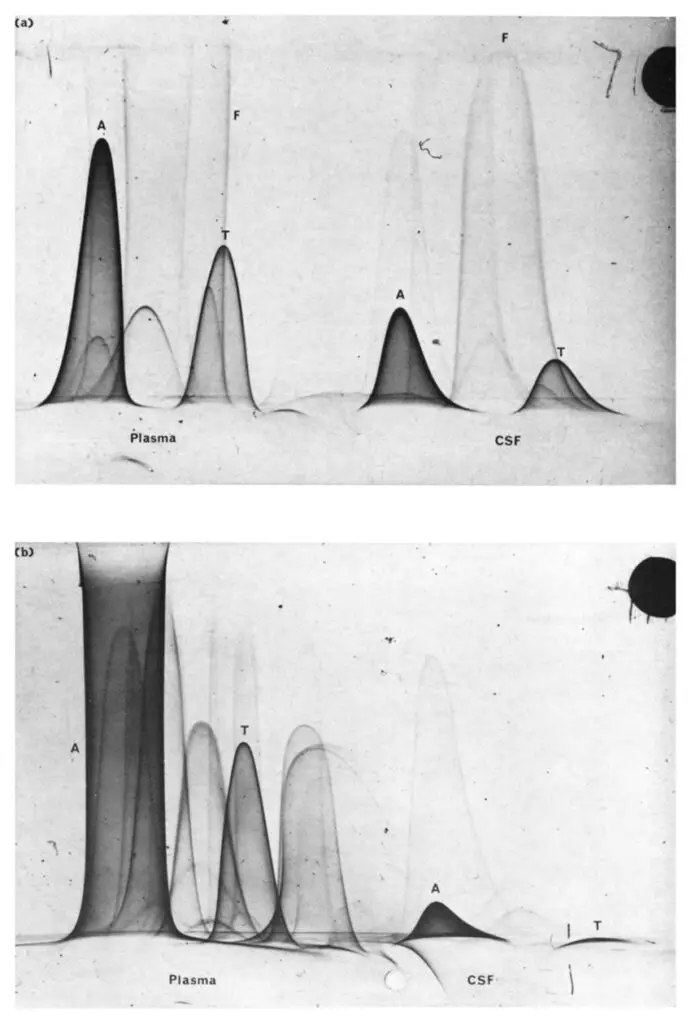
- Two-Dimensional Immunoelectrophoresis, also known as crossed immunoelectrophoresis, is a technique that allows for the quantitation and analysis of one or more proteins within a protein mixture. It is a modification of Laurell’s rocket electrophoresis and was first described by Ressler in 1960, with subsequent improvements made by Laurell, Clarke & Freeman, and Weeke.
- Unlike Laurell’s rocket electrophoresis, which focuses on the quantification of a single protein, two-dimensional immunoelectrophoresis enables the comprehensive analysis of protein mixtures. It provides a rapid and efficient means to determine the constituents of protein samples.
- The assay involves two distinct electrophoresis steps: a normal electrophoresis step followed by a rocket electrophoresis step. In the first step, the protein mixture is subjected to electrophoresis, leading to the separation of individual components. A section of the gel containing the separated antigens is then sliced and placed on a glass plate.
- Next, a layer of agar containing antiserum is poured onto the glass plate, adjacent to the gel slice. The gel slice, positioned towards the cathode, undergoes the rocket electrophoresis step in a direction perpendicular to the first run. As the electrophoresis proceeds, the antigens from the gel slice enter the agar containing antiserum, resulting in the formation of precipitin arcs at the zone of equivalence.
- The height of the precipitin arcs formed by standards can be utilized to determine the quantity of individual antigens present in the sample, similar to Laurell’s rocket electrophoresis. By comparing the heights of the arcs formed by the unknown samples to those of the standards, the concentrations of the corresponding proteins can be calculated.
- Two-dimensional immunoelectrophoresis provides a comprehensive approach to analyze protein mixtures, allowing for the quantitation of multiple proteins simultaneously. This technique enables researchers to gain insights into the composition and relative abundance of proteins in complex samples. By incorporating two distinct electrophoresis steps, it enhances the specificity and accuracy of protein analysis, making it a valuable tool in various fields such as clinical diagnostics, biomedical research, and proteomics.
Advantages
Two-Dimensional Immunoelectrophoresis, or crossed immunoelectrophoresis, offers several advantages that make it a valuable technique for protein quantification and analysis. Some of these advantages include:
- Quantification of Multiple Components: One of the primary advantages of two-dimensional immunoelectrophoresis is its capability to quantify different components of proteins within a mixture. By employing specific antibodies that interact with their corresponding antigens, the technique enables the simultaneous quantification of multiple proteins present in a sample. This feature allows researchers to gain a comprehensive understanding of the protein composition and relative abundance within complex mixtures.
- High Sensitivity: Two-dimensional immunoelectrophoresis is known for its high sensitivity. The use of antibody-antigen interactions enhances the specificity of the assay, resulting in precise detection and quantification of target proteins even at low concentrations. The sensitivity of the technique enables the detection of proteins present in trace amounts, making it particularly valuable when working with samples that may have limited protein content.
- Specificity: The technique relies on the specific binding between antibodies and their corresponding antigens. This specificity ensures that only the desired proteins are detected and quantified, minimizing background noise and interference from unrelated components. By leveraging the specificity of the antibody-antigen interactions, two-dimensional immunoelectrophoresis provides accurate and reliable results, enhancing the confidence in the obtained data.
- Versatility: Two-dimensional immunoelectrophoresis can be applied to various sample types, including biological fluids (such as serum, plasma, urine, and cerebrospinal fluid), tissue extracts, and other protein mixtures. This versatility allows researchers to explore protein composition and quantification in diverse biological and clinical samples. It provides a flexible tool that can be adapted to suit different research questions and experimental needs.
- Rapid Analysis: The technique offers relatively quick analysis of protein mixtures. While the specific time frame may vary depending on the experimental setup, two-dimensional immunoelectrophoresis generally provides rapid results compared to other protein quantification methods. This efficiency allows for efficient processing of multiple samples, making it suitable for high-throughput applications.
In summary, two-dimensional immunoelectrophoresis, or crossed immunoelectrophoresis, offers advantages such as the quantification of multiple protein components, high sensitivity, specificity through antibody-antigen interactions, versatility in sample types, and rapid analysis. These attributes make it a valuable technique in protein research, clinical diagnostics, and various fields where precise protein quantification and analysis are essential.
Applications of Immunoelectrophoresis
Immunoelectrophoresis has several applications in various fields, including clinical diagnostics, research, and immunology. Some of the key applications of immunoelectrophoresis are:
- Protein identification and quantification: Immunoelectrophoresis allows for the identification and approximate quantization of various proteins present in serum or other biological samples. It provides a valuable tool for studying protein composition and abnormalities.
- Gammopathies detection: Immunoelectrophoresis is widely used in the diagnosis of gammopathies, including monoclonal and polyclonal gammopathies. It helps in detecting abnormal proteins, such as monoclonal immunoglobulins (M-proteins) in serum or urine, which are indicative of conditions like multiple myeloma.
- Analysis of complex protein mixtures: Immunoelectrophoresis is useful for analyzing complex protein mixtures containing different antigens. It helps in separating and characterizing individual proteins within the mixture based on their electrophoretic mobility and reaction with specific antibodies.
- Diagnosis of immune-related diseases: Immunoelectrophoresis aids in the diagnosis and evaluation of therapeutic responses in various disease states affecting the immune system. It can be used to assess the presence or absence of specific proteins associated with immune deficiencies, autoimmune disorders, and other immune-related conditions.
- Antigen and antibody purity monitoring: Immunoelectrophoresis can be utilized to monitor the purity of antigens and antibodies in research and diagnostic settings. It helps in assessing the quality and specificity of these reagents, ensuring reliable experimental results.
- Qualitative analysis of M-proteins: Immunoelectrophoresis is an older method for the qualitative analysis of M-proteins in serum and urine. It aids in the detection and characterization of monoclonal gammopathies, providing valuable information for disease diagnosis and monitoring.
- Research in immunology: Immunoelectrophoresis continues to be used as a valuable research tool in immunology. It allows for the study of antigen-antibody interactions, determination of immunodominant epitopes, and investigation of immune responses.
The diverse applications of immunoelectrophoresis make it a versatile technique for protein analysis, disease diagnosis, and immunological research. Its ability to separate and characterize proteins based on their electrophoretic mobility and interaction with antibodies contributes to our understanding of various biological processes and pathological conditions.
FAQ
What is immunoelectrophoresis?
Immunoelectrophoresis is a laboratory technique that combines electrophoresis and immunodiffusion to separate and identify proteins based on their antigen-antibody interactions.
What are the different types of immunoelectrophoresis?
The main types of immunoelectrophoresis include radial immunodiffusion, rocket electrophoresis, crossed immunoelectrophoresis, immunofixation electrophoresis, and two-dimensional immunoelectrophoresis.
How does rocket electrophoresis work?
Rocket electrophoresis involves the migration of antigens through a gel matrix towards anode, forming an immunoprecipitation arc in the shape of a rocket. The height of the rocket is directly proportional to the antigen concentration.
What is radial immunodiffusion?
Radial immunodiffusion is a type of immunoelectrophoresis in which antigens diffuse radially outward from a well in a gel containing specific antibodies. The size of the precipitin ring formed is proportional to the antigen concentration.
What is crossed immunoelectrophoresis?
Crossed immunoelectrophoresis combines conventional electrophoresis with immunodiffusion. It involves two electrophoresis steps: a first step to separate antigens and a second step to detect and quantify them using specific antibodies.
What is immunofixation electrophoresis?
Immunofixation electrophoresis is a technique that combines electrophoresis with immunodiffusion and immunoprecipitation. It is used to identify and characterize abnormal proteins, such as monoclonal immunoglobulins.
What is two-dimensional immunoelectrophoresis?
Two-dimensional immunoelectrophoresis is a technique that combines two different electrophoresis steps to separate and quantify multiple proteins in a mixture. It provides a comprehensive analysis of protein composition.
How is immunoelectrophoresis used in clinical diagnostics?
Immunoelectrophoresis is used in clinical diagnostics to detect and quantify proteins, such as immunoglobulins, complement components, and acute-phase reactants. It can aid in the diagnosis and monitoring of various diseases.
What are the advantages of immunoelectrophoresis?
Immunoelectrophoresis offers advantages such as the ability to quantify specific proteins, sensitivity, specificity, versatility in sample types, and rapid analysis. It is a valuable tool in protein analysis and biomedical research.
What are the limitations of immunoelectrophoresis?
Immunoelectrophoresis has limitations such as the requirement for specific antibodies, potential cross-reactivity, limited resolution for complex mixtures, and the need for careful interpretation of results. It is essential to consider these factors when applying the technique.
