Advertisements
Table of Contents
- MHC molecules serve to bind peptides and deliver them to passing T lymphocytes for examination. In the case of selfpeptides, T cells with a TCR capable of binding with high affinity to the peptide–MHC complex have likely been eliminated by the mechanisms of central tolerance or rendered non-responsive by peripheral tolerance.
- In the event of a nonself peptide, however, a T cell clone containing a TCR capable of recognising that peptide attached to MHC is likely to exist.
- When an APC or target cell provides enough peptide–MHC complexes to occupy sufficient TCRs on the antigen-specific T cell to initiate the activation phase, an immune response develops.
- Consequently, the degree and timing of MHC molecule expression are critical in deciding whether an immunological response will be produced.
- When and where the MHC genes are expressed is largely determined by cytokines and other stimuli generated in the vicinity of the host cell.
- Depending on the type of host cell and the tissue in which it dwells, these stimuli can either be produced constitutively or triggered during an inflammatory or immunological response to injury, infections, or malignancies.
- For instance, chemicals in the walls of invading bacteria trigger macrophages to create TNF and lymphotoxins, whereas viral infection induces infected cells to make interferons (IFNs).
- The activation of transcription factors is triggered by the contact of these cytokines with particular receptors on the host cell.
- The active transcription factors reach the nucleus of the host cell and bind to five regulatory patterns in the DNA upstream of the MHC genes, either increasing or inhibiting the expression of the MHC genes.
- The expression of MHC class I and class II genes exhibits fundamental variances. MHC class I proteins are expressed on nearly all nucleated cells, but MHC class II expression is restricted to a few of cell types capable of presenting antigen to CD4 T cells, such as B cells, macrophages, Langerhans cells, follicular dendritic cells, and interdigitating cells.
- MHC class II also exists on thymic epithelial cells and functions in thymic selection processes that lead to tolerance. Despite these distinctions, it is now understood that the MHC class I and II genes share at least one key regulation route dependent on the SXY regulatory module.
1. The SXY–CIITA Regulatory System
- There are regulatory elements in the 5 upstream regions of the promoters of the MHC class I, β2m, and MHC class II α and β genes (and other accessory genes, such as the one encoding the invariant chain) that allow for their coordinated transcription.
- Thus, the proteins required for the formation of entire MHC class I and II molecules as well as other proteins involved in antigen presentation are synthesised simultaneously, despite the fact that some of the genes may be located outside the MHC.
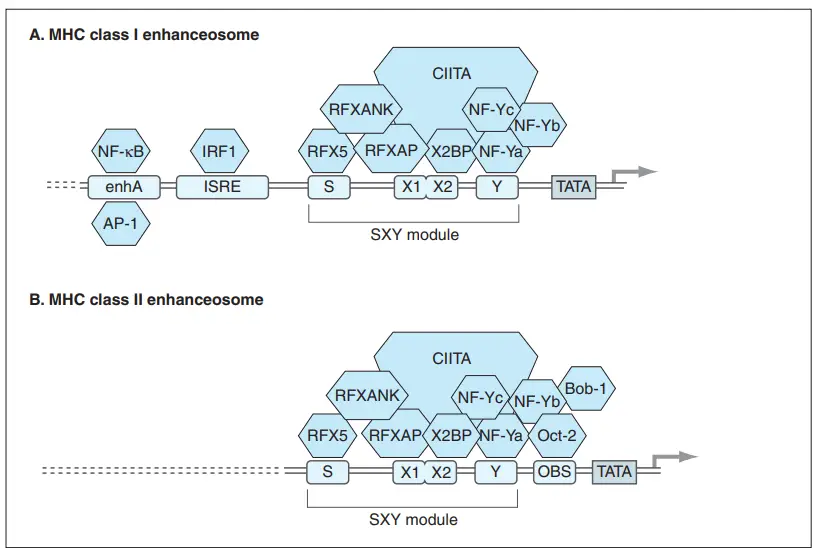
- One of the most prominent regulatory motifs is the SXY module, which consists of the S box (also called as the W/S box), the X box (containing tandem X1 and X2 sequences), and the Y box (beginning at the 5 end of the regulatory area and progressing 3 toward the promoter).
- Historically, these regulatory motifs were determined by comparing the sequences of the promoter regions of the murine and human MHC class II genes and by evaluating the effects of mutations on the expression of MHC class II genes.
- The regulatory DNA sequences of the S, X, and Y boxes are conserved among MHC genes and interact with at least four or five different multi-subunit factors (and hence at least 8–10 different polypeptides) to generate a complex that regulates the expression of MHC genes.
- It has been demonstrated that precise spacing (i.e., the correct number of helical turns) between the X and Y boxes is essential for the binding of these regulatory proteins. In fact, the X and Y boxes are separated by a 19–20 bp stretch of DNA whose sequence is variable but whose length is consistent across all species studied to date.
- Each of the three components of the SXY module is held together by at least one component of a massive cooperative binding complex. RFX binds poorly to the S box and firmly to the X1 element of the X box. The components of RFX are the RFX5 protein, the RXF-associated protein (RFXAP), and the RFX ankyrin repeat protein (RFXANK).
- X2 binding protein (X2BP), a complex of CREB (cAMP-responsive element binding) transcription factors, binds the X2 element of the X box. X2BP appears to be necessary for RFX DNA binding in vivo.
- Scientists hypothesise that the Y box may play a role in maintaining the RFX–X2BP–DNA complex. The Y box is bound by the heterotrimeric DNA binding factor NF-Y, which consists of the NF-Ya, NF-Yb, and NF-Yc subunits. The Y box is the SXY molecule’s most conserved component.
- It is believed that the NF-Y complex interacts with local chromatin structures to facilitate transcription. We know the Y box is crucial because individuals with mutations in the Y box express significantly less MHC class I on a constitutive basis.
- In the majority of cells, the RFX, X2BP, and NF-Y binding factor proteins are constitutively expressed and occupy the X and Y boxes of the SXY module. This complex assembly of RFX/X2BP/NF-Y on the SXY module serves as a platform for the class II transactivator (CIITA) protein.
- CIITA was formerly believed to be expressed primarily in MHC class II-positive cells (thus its name), but it was recently shown that it also influences MHC class I transcription. Low concentrations of CIITA are required for optimum constitutive and inducible production of MHC class I.
- CIITA does not bind directly to promoter DNA, but rather to the entire RFX/X2BP/NF-Y complex. This complex, multi-protein transactivator structure located on an active promoter is known as an enhanceosome.
- CIITA is even more crucial for transcription of MHC class II. Both constitutive and inducible MHC class II expression are virtually eliminated in the absence of significant amounts of CIITA.
- The recruitment of CIITA to the RFX/X2BP/NF-Y complex is essential for MHC class II gene transcription in APCs.
- Consistent with CIITA’s function as the limiting factor for MHC class II expression, mature plasma cells that do not express MHC class II also do not express CIITA. Intriguingly, while CIITA/ mice lack any expression of MHC class II on their cells, humans lacking CIITA exhibit a low level of MHC class II expression, suggesting that distinct compensatory mechanisms may function between species.
- In B cells, CIITA may also interact with Bob-1, also known as OBF-1 (octamer binding factor-1) or OCA-B, a non-DNA-binding regulatory protein. Bob-1, which is uniquely expressed in B cells, interacts with the transcription factor Oct-2 to trigger the transcription of genes that are essential for the growth and activation of B cells.
- Oct-2 binds to the OBS motif found between the Y box and the TATA box. According to several researchers, CIITA and Bob-1 work synergistically to induce the elevated levels of MHC class II gene transcription observed in the majority of B cells.
- CIITA deficiency is one cause of bare lymphocyte syndrome, an autosomal recessive human immunodeficiency. In an attempt to tissue type these patients, histologists were unable to find MHC class II antigens on the patient specimens isolated for study, in this case a mixed population of B and T cells.
- This condition was found before it was understood that B cells can operate as APCs, hence it may have been more appropriately dubbed “bare APC syndrome.” Recent research has demonstrated that genetic abnormalities in each subunit of the RFX factor can also result in bare lymphocyte syndrome.
2. TNF- And IFN-Induced Expression Of Mhc Class I
- It is believed that RFX expression regulates the low levels of constitutive MHC class I on nearly all cell types. Notwithstanding, TNF or αIFN, -β, or -γ can trigger a further rise in MHC class I expression.
- During both innate and adaptive immune responses, the invasion of host tissues by gram-negative bacteria causes the generation of TNF and lymphotoxins by activated macrophages. In the promoters of MHC class I genes, well upstream of the SXY module is a region called Enhancer A, which contains many motifs for nuclear transcription factors [especially NF- kB and activating protein-1 (AP-1)].
- NF- kB and AP-1 are activated in response to TNF and bind to their respective motifs in Enhancer A and the Y box of the SXY module, thereby driving MHC class I transcription.
- During viral infections, another regulatory element known as the interferon-stimulated response element (ISRE) plays a significant role. IFNα is produced by cells of the innate immune system in response to viral infection, while IFNβ is produced by fibroblasts.
- NK cells, CD8 T cells, and CD4 Th1 cells that are activated during an immune response release IFNγ. The IFNs act to mobilise members of the IRF (interferon regulatory factor) family of regulatory molecules.
- Some IRF members can be induced by interferon, but others are constitutively expressed. Mobilized IRF molecules bind to the ISRE regulatory motif between Enhancer A and the SXY module in the promoters of all MHC class I genes.
- IRF binding to the ISRE increases the activation of nuclear transcription factors that boost MHC class I transcription, hence enhancing the presentation of viral peptides by infected cells. Thus, CD8 T cells kill more of these targets, and the infection is confined more quickly.
- While many IRFs that bind to the ISRE activate nuclear transcription factors that enhance MHC class I transcription, the ISRE-binding protein ICSBP appears to negatively regulate MHC class I transcription (interferon consensus sequence binding protein).
- The ISRE route may be responsible for the fact that, in the absence of CIITA, MHC class I expression is reduced but not eliminated. ISRE sequences are absent from MHC class II genes, hence IFNγ cannot directly trigger their expression via this mechanism.
- In normal APCs, IFNγ stimulates the production of CIITA mRNA, which interacts with the RFX/X2BP/NF-Y complex to upregulate MHC class II transcription.
3. Expression Of Mhc Class Ib Genes
- Many class Ib genes are not universally expressed like MHC class I genes. In class Ib genes, nucleotide sequence changes in the promoter regions have been found to be the molecular cause of the varying expression levels.
- For instance, the HLA-G gene’s promoter has a missing or damaged ISRE site, preventing IFNγ from inducing transcription of this gene.
- The HLA-G promoter has been modified in a way that prevents NF-kB and CIITA from inducing gene expression by changing the NF-kB binding motifs in Enhancer A and the X2 and Y boxes in the SXY module.
- It is true that HLA-G molecules, which are potential mediators of maternal-fetal tolerance, are expressed almost exclusively in the highly specialised trophoblast cells of the pregnant uterus.
- Element(s) regulating expression in these contexts are being sought. When the sequence of the HLA-E promoter is altered in a similar fashion, transcription of HLA-E is inhibited in response to NF- kB.
- The ISRE is not directly involved in IFN-induced HLA-E synthesis; rather, a STAT1 (signal transducer and activator of transcription 1) binding site is located upstream of the ISRE.
- CIITA also has the ability to induce HLA-E by way of the SXY module. HLA-ability F’s to induce NF-kB seems to depend in part on interactions with sequences in or near the ISRE. IFN activates HLA-F expression through the ISRE, and CIITA activates it through the SXY module.
4. Other Regulatory Pathways Governing Mhc Class II Gene Expression
- TGFβ, IL-4, IL-13, IL-10, and IFNβ are only some of the cytokines that can affect MHC class II expression.
- By reducing CIITA mRNA expression, TGF counteracts the inducible IL-2 response and preserves MHC class II expression. IL-4 and IL-13 elevate MHC class II expression on various APCs, while IL-10 and IFNβ block IFNγ-mediated MHC class II expression upregulation in a cell-type-specific manner.
