Table of Contents
What is Mucosa Associated Lymphoid Tissues (MALT)?
- Mucosa-associated lymphoid tissue (MALT) refers to a diffuse system of small concentrations of lymphoid tissue found in various submucosal membrane sites of the body. These sites include the gastrointestinal tract, nasopharynx, thyroid, breast, lung, salivary glands, eye, and skin. MALT is an essential component of the immune system and is strategically located to encounter antigens that pass through the mucosal epithelium.
- The lymphoid tissue within MALT is populated by various immune cells, including T cells, B cells, plasma cells, and macrophages. These cells play a crucial role in immune responses that occur at mucous membranes. For example, B cells produce antibodies, including immunoglobulin A (IgA), which provides local immunity and protection against pathogens. T cells are involved in cell-mediated immune responses.
- In the case of intestinal MALT, specialized cells called M cells are present. These M cells sample antigens from the intestinal lumen and deliver them to the underlying lymphoid tissue, initiating immune responses. This process helps in the recognition and elimination of harmful pathogens.
- The presence of MALT in various mucosal sites reflects the importance of these tissues in defending the body against microbial invasion. The majority of lymphoid tissue in the human body, comprising over 50%, is located within the lining of the respiratory, digestive, and genitourinary tracts. Additionally, small concentrations of lymphoid tissue can be found in other sites such as the thyroid, breast, lung, salivary glands, eye, and skin.
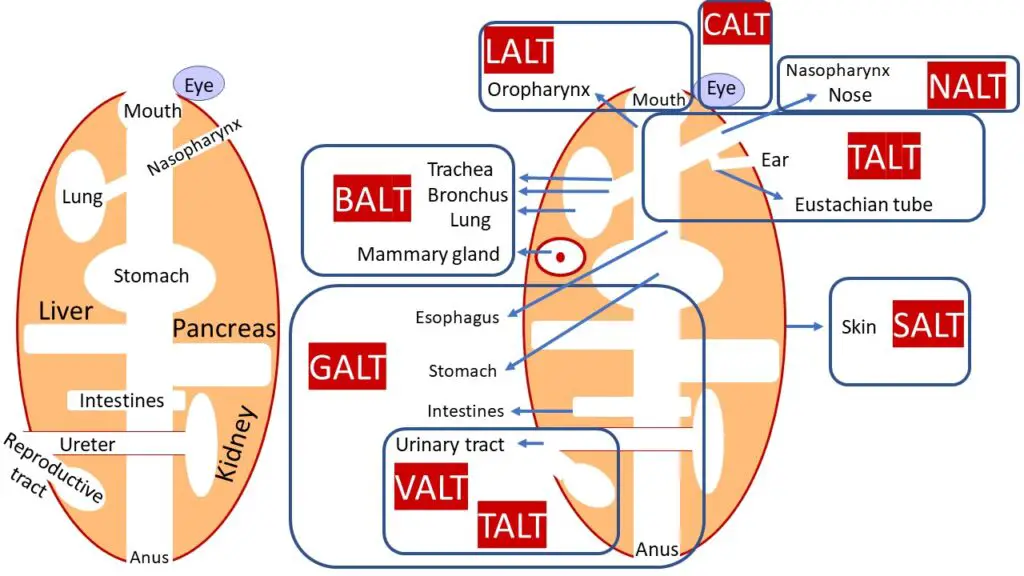
- Depending on their specific location, MALT can be further categorized into different subtypes. For instance, nasal-associated lymphoid tissue (NALT) refers to lymphoid tissue present in the nasal cavity, while gut-associated lymphoid tissue (GALT) refers to lymphoid tissue found in the gastrointestinal tract. Similarly, bronchus-associated lymphoid tissue (BALT) refers to lymphoid tissue associated with the bronchi, and there is also lymphoid tissue associated with the genitourinary system.
- The study of immune responses that occur at mucous membranes, including those involving MALT, falls within the field of mucosal immunology. Understanding the functioning and responses of MALT is crucial for comprehending the body’s defense mechanisms at mucosal surfaces and developing strategies to combat infections and diseases related to these areas.
Features of Mucosa Associated Lymphoid Tissues (MALT)
Mucosa-associated lymphoid tissue (MALT) exhibits several distinctive features:
- Lymphocyte Residence: Approximately half of the immune system’s lymphocytes are found within MALT. These lymphocytes, including T cells and B cells, are strategically located along the mucosal tissue surfaces of various organs and systems in the body.
- Broad Distribution: MALT is not limited to a single anatomical site. While it primarily exists in the mucosal tissues of the respiratory, digestive, and genitourinary tracts, it can also be found in other locations. Examples include conjunctiva-associated lymphoid tissue (CALT) in the eyes, lacrimal duct-associated lymphoid tissue (LDALT), larynx-associated lymphoid tissue (LALT), and salivary duct-associated lymphoid tissue (DALT).
- IgA Secretion: One of the primary functions of MALT is to generate and secrete immunoglobulin A (IgA) antibodies across mucosal surfaces. IgA plays a crucial role in providing local immunity and protection against pathogens at mucosal sites. These antigen-specific reactions that lead to IgA production are typically Th2-dependent, but Th1 and cytotoxic T-cell-mediated responses can also occur, contributing to immune tolerance.
- Effector and Inductive Sites: Functionally, MALT can be divided into effector sites and inductive sites. Inductive sites are locations where immune responses are initiated and include gut-associated lymphoid tissue (GALT), bronchus-associated lymphoid tissue (BALT), nasal-associated lymphoid tissue (NALT), CALT in certain animals like mice, dogs, and baboons, as well as DALT in cynomolgus macaques. These sites serve as sites of antigen encounter and immune activation.
MALT’s unique features highlight its role in mucosal immunity, allowing it to mount specific immune responses at the mucosal surfaces of different tissues. By generating and secreting IgA antibodies and facilitating various immune reactions, MALT contributes to the defense against pathogens and the maintenance of immune homeostasis in the mucosal environment.
Examples of Mucosa Associated Lymphoid Tissues (MALT)
Mucosa-associated lymphoid tissue (MALT) can be found in various locations throughout the body. Some examples of MALT include:
- Gut-Associated Lymphoid Tissue (GALT): GALT is located in the digestive tract and includes structures such as the tonsils, adenoids, Peyer’s patches in the small intestine, and the appendix. These lymphoid tissues protect against pathogens and initiate immune responses to antigens encountered in the gastrointestinal tract.
- Bronchus-Associated Lymphoid Tissue (BALT): BALT is present in the lungs and bronchial passages. It consists of lymphoid follicles and plays a role in initiating immune responses to airborne pathogens and antigens.
- Nasopharynx-Associated Lymphoid Tissue (NALT): NALT is situated in the nasal and nasopharyngeal regions. It serves as the immune system of the nasal mucosa and protects against airborne viruses and other infectious agents.
- Conjunctiva-Associated Lymphoid Tissue (CALT): CALT is located in the conjunctiva of the eye. It helps to defend against pathogens and triggers immune responses in the ocular surface.
- Lactating Mammary Gland-Associated Lymphoid Tissue (Mammary MALT): Mammary MALT is found in the breast tissue during lactation. It contributes to the local immune defense of the mammary glands and protects against potential infections in nursing mothers.
These are just a few examples of mucosa-associated lymphoid tissues (MALT) found in different parts of the body. Each site has specialized structures and functions to provide immune protection and response to antigens encountered in specific mucosal surfaces.
Structure of MALT
The structure of mucosa-associated lymphoid tissue (MALT) shares common features across various mucosal sites. Regardless of the specific location, all mucosal lymphoid tissues consist of several basic compartments.
- Follicles: MALT contains lymphoid follicles, which are organized clusters of immune cells. These follicles are primarily composed of B cells, surrounded by T cells. Within the follicles, there may be germinal centers where B cells undergo proliferation, maturation, and differentiation in response to antigen stimulation. The germinal centers play a crucial role in the production of specific antibodies.
- Interfollicular regions: These regions lie between the lymphoid follicles and are characterized by a dense population of T cells. The interfollicular regions serve as sites for interactions between T cells and antigen-presenting cells (APCs), promoting immune responses and cell-mediated immunity.
- Subepithelial dome regions: These regions are present beneath the epithelial layer of the mucosa and are particularly prominent in the gut-associated lymphoid tissue (GALT). Subepithelial dome regions contain specialized epithelial cells called M cells, which play a crucial role in antigen uptake from the mucosal surface. M cells transfer the captured antigens to underlying APCs, initiating immune responses within MALT.
- Follicle-associated epithelium: This specialized epithelial layer surrounds the lymphoid follicles. It is found in specific regions such as Peyer’s patches in the gut. The follicle-associated epithelium consists of M cells, which facilitate the transport of antigens from the lumen of the mucosal surface to the underlying immune cells. This transportation of antigens allows for efficient immune surveillance and response.
Overall, the structure of MALT exhibits a highly organized arrangement of lymphoid follicles, interfollicular regions rich in T cells, subepithelial dome regions with M cells, and follicle-associated epithelium. These structural components ensure effective immune surveillance and responses at mucosal surfaces throughout the body.
1. Gut-Associated Lymphoid Tissue (GALT)
- Gut-associated lymphoid tissue (GALT) is an essential component of mucosa-associated lymphoid tissue (MALT) and is critical in the immune system’s defense against intruders in the gut.
- The gut mucosal surface is thin and acts as a permeable barrier to food absorption. However, because it is thin and permeable, it is prone to infection. The majority of infectious pathogens that enter the human body enter through the gut. As a result, the GALT plays an important role in defending the body against harmful infections.
- GALT’s huge population of plasma cells is one of its distinguishing traits. Plasma cells are in charge of antibody production. Surprisingly, the quantity of plasma cells in GALT exceeds that of the spleen, lymph nodes, and bone marrow combined. This abundance of plasma cells demonstrates GALT’s functional role in the body’s defense processes. GALT accounts for over 70% of the immune system’s weight, showing its significant contribution to total immunological function.
- GALT is made up of lymphoid complexes that include epithelial cells, antigen-presenting cells (APCs), and intraepithelial lymphocytes. These complexes are strategically placed throughout the digestive tract, such as Peyer’s patches in the terminal ileum.
- M cells are specialized epithelial cells found in GALT. M cells are closely related to APCs and play an important role in antigen sampling within the gut. M cells collect antigens from the intestinal lumen and transfer them to lymphoid tissue via APCs. This mechanism makes it easier to recognize and respond to any pathogens or toxic substances in the stomach.
- The presence of M cells and antigen-presenting cells in GALT enables for good antigen surveillance and response. GALT helps to preserve the integrity of the gut mucosa and guard against invading pathogens by starting immunological responses and creating antibodies, adding to the overall health of the immune system.
Structure of Gut-Associated Lymphoid Tissue (GALT)
- The gut-associated lymphoid tissue (GALT) is distributed throughout the intestine, covering a substantial area of approximately 260-300 square meters. The intestinal mucosa is structured to increase the surface area for absorption and is composed of finger-like projections called villi. These villi are covered by a monolayer of epithelial cells, which form a barrier between the GALT and the intestinal lumen and its contents. To protect these epithelial cells from the acidic pH of the gut, they are coated with a layer of glycocalyx on their luminal surface.
- The intestinal glands within the crypts of the mucosa continually produce new epithelial cells derived from stem cells, ensuring the constant regeneration of the epithelium. The turnover time of these epithelial cells is less than one week. While conventional enterocytes are the dominant cell type within the crypts, Paneth cells are also present. Located at the bottom of the crypts, Paneth cells release antibacterial substances, including lysozyme, and are believed to play a role in infection control.
- Beneath the epithelial layer, there is a layer of loose connective tissue known as the lamina propria. This tissue contains lymphatic circulation connected to the mesenteric lymph nodes. Both GALT and mesenteric lymph nodes serve as sites where the immune response is initiated due to the presence of immune cells within the epithelial cells and lamina propria.
- In addition to the general distribution of GALT throughout the intestine, specific structures are part of the GALT. These include Peyer’s patches in the small intestine, isolated lymphoid follicles dispersed throughout the intestine, and the appendix in humans. Various lymphoid tissues act as interfaces between the immune system and incoming antigens, including the tonsillar ring of Waldeyer, small lymphoid aggregates in the esophagus, lymphoid tissue in the stomach, lymphoid aggregates in the appendix and large intestine, and intraepithelial lymphocytes interspersed within the epithelial layer of mucosal surfaces.
- GALT can be divided into two categories based on their structure and associated function. Organized GALT consists of follicles, such as Peyer’s patches, mesenteric lymph nodes, and the more organized appendix. Its primary function is to induce an immune reaction. Diffuse GALT, on the other hand, consists of scattered T and B cells, macrophages, eosinophils, basophils, and mast cells, primarily located in the lamina propria. This part of GALT is composed of mature effector cells ready to carry out their immune functions.
- GALT has been studied in various animal species, including adult eastern grey kangaroos, tammar wallabies, stripe-faced dunnarts, and red-tailed phascogales. The development of GALT has also been described in several marsupial species, such as tammar wallabies, stripe-faced dunnarts, and red-tailed phascogales.
What are Peyer’s patches?
- Peyer’s patches are specialized structures that consist of lymphoid cells projecting into the lumen of the gut. They play a crucial role in initiating the immune response within the gut.
- These patches form a subepithelial dome, which contains a significant number of B cell follicles with their germinal centers. Additionally, there are T cell regions, although in smaller numbers, and dendritic cells present in this area.
- The subepithelial dome is separated from the intestinal lumen by a layer of follicle-associated epithelium. This epithelium comprises ordinary intestinal epithelial cells and a small number of specialized epithelial cells known as microfold cells or M cells.
- Unlike enterocytes, which are the predominant epithelial cells in the gut, M cells have a folded luminal surface instead of microvilli. They do not secrete digestive enzymes or mucus, and they lack a thick surface layer called glycocalyx. This unique structure allows M cells to come into direct contact with the microbiota and antigens present in the gut’s contents.
- By interacting with antigens and sampling them from the gut lumen, M cells play a crucial role in initiating immune responses and facilitating the transport of antigens to the underlying lymphoid tissue within Peyer’s patches. This process is essential for the development of adaptive immune responses against pathogens and commensal microorganisms in the gut.
Functions of GALT
GALT (Gut-Associated Lymphoid Tissue) has various critical roles in the immune system.
- B-Lymphocytes: Plasma B cells in the gut’s lamina propria produce secretory IgA (sIgA) antibodies. Transcytosis transports these antibodies through the epithelial layer into the intestinal lumen. The interplay of B cells, antigen-presenting dendritic cells (DCs), and follicular T helper cells (Tfh) in the germinal centers of Peyer’s patches promotes sIgA production. Secretory IgA coats both commensal and pathogenic intestinal bacteria, reducing motility and preventing them from coming into direct touch with the intestinal epithelium and the host immune system. In addition, secreted IgA binds to bacterial toxins, mitigating their damaging effects.
- T-Lymphocytes: In the GALT, naive CD4+ T cells differentiate into Treg (regulatory T cells), Th1, Th2, Th17, or Tfh cells. This differentiation happens by the presentation of antigens generated from the gut microbiota by antigen-presenting cells in Peyer’s patches such as dendritic cells or M cells. The interaction of DCs and T cells in the GALT results in the formation of immunosuppressive Treg cells, which aid in the maintenance of tolerance to ingested dietary antigens. Furthermore, commensal microbiota can trigger an immunological response that protects the host’s intestinal tissue against immune cell reactions. T cell population balance in the GALT is critical for maintaining intestinal homeostasis and preventing pathological harm to the host.
- Intraepithelial Lymphocytes (IELs): IELs are a huge population of T lymphocytes that live in the intestine’s epithelial layer. Traditional IELs, which are produced from naive T cells that encounter antigens in the periphery, express gut-tropic molecules and function as tissue-resident effector memory T cells. They respond quickly to infections through cytolytic activity and the release of cytokines. The bulk of IELs in the intestinal epithelium are unconventional IELs, principally gamma-delta () T cells. After leaving the thymus and encountering antigens in the GALT, they develop their effector program. T cells are involved in long-term immunological memory in barrier tissues such as the intestinal epithelium.
- Innate Lymphoid Cells (ILCs): ILCs are a recently identified class of innate immune cells that play an important role in mucosal immunity and homeostasis. They have the ability to release immunoregulatory cytokines quickly and connect with other immune cells. At mucosal surfaces, such as the GALT, several subsets of ILCs, such as NK cells, ILC1s, ILC2s, ILC3s, and LTi cells, are common.
- Innate Immunity: The GALT also has innate immunity, which responds quickly to microbial non-self markers. Pattern recognition receptors (PRRs), which are located on a variety of immunological and epithelial cells, identify conserved microbial patterns or nucleic acids. Under normal settings, macrophages, which are produced from monocytes and found in the lamina propria, contribute to tissue homeostasis but can become proinflammatory effector cells during infection or inflammation. Dendritic cells (DCs) in the GALT direct the development of B cells in follicles and help Treg and traditional IELs differentiate into mature effector cells in the gut.
Overall, the GALT acts as a sophisticated immune system within the gut, maintaining tolerance to ingested antigens while defending against viruses and toxic substances. It involves the interplay of B cells, T cells, IELs, ILCs, macrophages, and dendritic cells, all of which play important roles in regulating immunological responses in the gut.
2. Nasopharynx-Associated Lymphoid Tissue (NALT)
- Nasopharynx-Associated Lymphoid Tissue (NALT) is a part of the mucosa-associated lymphoid tissue (MALT) found in the nasal mucosa. It serves as the immune system of the nasal passages, protecting the body from airborne viruses and other infectious agents.
- In humans, NALT is considered analogous to Waldeyer’s ring, which includes the tonsils and other immune cells underlying the throat and nasal passages.
- The structure of NALT is similar to lymph nodes, but it lacks a capsule and lymphatics. It consists of follicles composed primarily of B cells, surrounded by T cells and the germinal center. The germinal center is the site where B cells undergo antigen-dependent proliferation.
- Antigens and foreign particles that enter the nasal passages are trapped within the deep crypts of the lympho-epithelium. From there, they are transported to the lymphoid follicles within NALT. This allows for the interaction between the antigens and immune cells, leading to an immune response against potential pathogens.
- Overall, NALT plays a crucial role in the defense against airborne infections by capturing antigens and activating the immune system within the nasal mucosa.
Structure of Nasopharynx-Associated Lymphoid Tissue (NALT)
- The structure of Nasopharynx-Associated Lymphoid Tissue (NALT) in mice is localized on the cartilaginous soft palate of the upper jaw. It is situated bilaterally on the posterior side of the palate. NALT primarily consists of lymphocytes, with distinct zones enriched in T cells and B cells.
- The follicle-associated epithelium (FAE) within NALT contains specialized epithelial cells called M cells, which play a role in antigen intake from the mucosa. Additionally, some erythrocytes, dendritic cells, and macrophages are present in NALT. In certain areas, lymphatic vessels and high endothelial venules (HEVs) can be found.
- The cellular composition of NALT includes a roughly equal number of T cells and B cells. Among the T-cell population, there are approximately three to four times more CD4+ T cells than CD8+ T cells. The majority of T cells in NALT possess αβ T-cell receptors (TCR), while only a small portion have γδ TCR. CD4+ T cells in NALT are primarily in a naive state, characterized by high expression of CD45RB. B cells, on the other hand, are predominantly in an unswitched state, exhibiting the sIgM+ IgD+ phenotype.
- Overall, the structure of NALT in mice includes distinct lymphocyte zones, specialized epithelial cells, and the presence of dendritic cells and macrophages. The cellular composition is balanced between T cells and B cells, with CD4+ T cells in a naive state and B cells primarily in an unswitched state.
Functions of NALT
- The Nasopharynx-Associated Lymphoid Tissue (NALT) in mice serves a crucial function as a strategic defense against incoming pathogens, particularly those that are inhaled. It acts as the primary site for the recognition and elimination of inhaled pathogens, playing a key role in inducing both mucosal and systemic immune responses. Similar to Peyer’s patches in the small intestine, NALT serves as an inductive site for the mucosa-associated lymphoid tissue (MALT).
- Upon intranasal immunization or pathogen recognition, lymphocytes within NALT undergo proliferation and differentiation. They begin to produce various cytokines, including IFN-γ, type I interferons, IL-2, IL-4, IL-5, IL-6, and IL-10, depending on the specific immunizing agent or adjuvant used. B cells within NALT undergo isotype switching and start producing antigen-specific antibodies, primarily IgM, IgG, and notably, IgA. Activated B cells have the ability to migrate throughout the body, particularly to the respiratory and genitourinary tracts, as they express chemokine receptors CCR10 and α4β1-integrin. This allows them to provide localized immune protection in these regions. Additionally, long-lasting memory T and B cells are established within NALT following immunization, contributing to durable immune responses.
- Overall, the function of NALT is to serve as the first line of defense against inhaled pathogens, initiating immune responses, promoting antibody production, and establishing immune memory. It plays a crucial role in both mucosal and systemic immunity, contributing to the overall protection of the respiratory and other relevant mucosal surfaces.
3. Bronchus-Associated Lymphoid Tissue (BALT)
- Bronchus-Associated Lymphoid Tissue (BALT) is a type of tertiary lymphoid structure that is part of the mucosa-associated lymphoid tissue (MALT). It consists of lymphoid follicles located in the lungs and bronchus, playing a crucial role as an effective priming site for both mucosal and systemic immune responses.
- Structurally, BALT resembles other lymphoid tissues found in the gut, such as Peyer’s patches. It primarily consists of aggregates of lymphocytes organized into follicles, which can be found in all lobes of the lung as well as along the main bronchi. Within the follicles, the majority of lymphocytes are B cells, which play a significant role in the immune response.
- Antigen sampling within BALT is carried out by the epithelial cells that line the surface of the mucosa. Additionally, M cells are present within BALT, which transport antigens to the underlying antigen-presenting cells (APCs) and lymphocytes. This process allows for the efficient uptake and presentation of antigens, facilitating immune recognition and response.
- By serving as a site for antigen sampling and immune activation, BALT plays a crucial role in initiating and coordinating immune responses within the lungs and bronchial regions. It contributes to the defense against respiratory pathogens and helps establish both local and systemic immune protection. The presence of BALT highlights the importance of mucosal immune responses in the respiratory system and underscores its significance in overall immune defense.
Structure of Bronchus-Associated Lymphoid Tissue (BALT)
- The structure of Bronchus-Associated Lymphoid Tissue (BALT) varies among different mammal species, particularly in terms of its maintenance and inducibility. In certain species like rabbits or pigs, BALT is a normal component of the lungs and bronchus. However, in mice and humans, it is only observed following infection or inflammation, and thus referred to as inducible BALT (iBALT). Despite these differences, BALT and iBALT are structurally and functionally similar, and for the purposes of this article, the term BALT will be used to encompass both structures.
- BALT is typically located along the bifurcations of the upper bronchi, situated directly beneath the epithelium and often positioned between an artery and a bronchus. It can also be found in perivascular, peribronchial, and even interstitial areas in the lower airways of the lung. To be classified as BALT, it must exhibit a structured accumulation of lymphocytes and other immune cells.
- Within BALT, there are lymphoid follicles that contain germinal centers, where B cells are predominantly surrounded by T-cell areas. The interfollicular T-cell area is populated by dendritic cells that present antigens to T cells, while follicular dendritic cells can be found within the germinal centers. Germinal centers in BALT consist of CD4+ T helper lymphocytes, and they are also present in the interfollicular area, whereas CD8+ T cells are mainly found in the interfollicular region. High endothelial venules (HEVs) are another characteristic feature of BALT, located at the T/B-cell interface. These specialized blood vessels enable the recruitment of naive T cells into BALT and serve as the sole entry point for lymphocytes, which can then leave via efferent lymphatic vessels.
- In some species, such as mice, the epithelium above BALT may contain M cells, similar to those found in the dome epithelium of Peyer’s patches, although the presence of dome epithelium is not typical for BALT.
- The formation of BALT in mice requires the presence of interleukin-17 (IL-17), as well as molecules such as VCAM-1, PNAd, and LFA-1. Notably, the development of secondary lymphoid organs like lymph nodes and Peyer’s patches typically depends on lymphotoxin-α (LTα), whereas BALT formation is LTα-independent. Additionally, impaired function of regulatory T cells (Treg cells) may contribute to the formation of BALT.
- Overall, the structure of BALT consists of organized lymphoid follicles, germinal centers, T-cell areas, dendritic cells, and specialized blood vessels, all of which contribute to its immune function within the bronchial and lung regions.
Function of Bronchus-Associated Lymphoid Tissue (BALT)
- The function and purpose of Bronchus-Associated Lymphoid Tissue (BALT) are not fully understood. It remains unclear whether its production is a natural aspect of the immune response or if it is a pathological phenomenon that should be suppressed.
- However, BALT does contribute to the efficient priming of adaptive B-cell and T-cell responses against airborne antigens. Dendritic cells play a crucial role in maintaining and supporting the function of BALT. Inducible BALT (iBALT) is formed in response to infections such as influenza, reaching its maximum size approximately 1 to 2 weeks after infection before gradually declining. It is important to note that immune responses initiated in iBALT are delayed compared to the immune response in the draining lymph nodes, due to the time required for the formation of iBALT.
- In some chronic diseases, iBALT may play a role in the underlying pathology. BALT can also be induced in fetal lungs following conditions like chorioamnionitis or intrauterine pneumonia. Additionally, there is evidence suggesting that cigarette smoke exposure can induce the formation of BALT in humans and rats. Other stimuli, such as inflammation caused by rheumatoid arthritis or other autoimmune lung diseases, as well as mechanical injury caused by dust particles, can also trigger the development of BALT.
- In summary, while the exact function and purpose of BALT are still being investigated, it is involved in the efficient initiation of adaptive immune responses against airborne antigens and relies on dendritic cells for its maintenance and function. It is formed in response to infections and can be implicated in the pathology of certain chronic diseases. Furthermore, various stimuli, including inflammation and mechanical injury, can induce the formation of BALT.
Functions of MALT
The mucosa-associated lymphoid tissue (MALT) plays several important functions in the immune system.
- One of its main functions is to initiate immune responses to specific antigens encountered along all mucosal surfaces. This includes the diffuse lymphoid tissue found throughout mucosal surfaces. MALT is responsible for the production and transport of immunoglobulin A (IgA) across the mucosal epithelium. IgA is an antibody that plays a crucial role in mucosal immunity by neutralizing pathogens and preventing their attachment to mucosal surfaces.
- GALT (gut-associated lymphoid tissue), a specific subset of MALT, has a primary role in protecting the body against microbes that enter via the intestinal tract. The GALT consists of various components, including Peyer’s patches and isolated lymphoid follicles, which are specialized structures in the gut that contain immune cells.
- M cells are specialized epithelial cells found in mucosal surfaces, particularly in the gut-associated lymphoid tissue. They play a crucial role in antigen uptake by taking up foreign molecules and passing them to underlying antigen-presenting cells (APCs). These APCs then present the antigens to T cells through major histocompatibility complex (MHC) molecules. This interaction between APCs and T cells helps activate B cells, which produce antibodies, and both T and B cells can migrate to other parts of the gastrointestinal (GI) tract, salivary glands, and other MALT sites. This migration allows them to protect these surfaces from invasion by the same microbes encountered.
- Furthermore, the presence of nasal-associated lymphoid tissue (NALT) and bronchus-associated lymphoid tissue (BALT) in the airways suggests their direct involvement in handling airborne microbes. These specialized lymphoid tissues in the nasal and bronchial regions are strategically positioned to recognize and eliminate inhaled pathogens, contributing to local and systemic immune responses.
- MALT tissues constitute the mucosal immune system, which can function independently of the systemic immune system. They are an essential aspect of immunity, providing specialized protection at mucosal surfaces. By initiating immune responses, producing specific antibodies, and facilitating cell migration, MALT plays a critical role in defending the body against pathogens encountered along mucosal surfaces.
References
- Randall TD. Bronchus-associated lymphoid tissue (BALT) structure and function. Adv Immunol. 2010;107:187-241. doi: 10.1016/B978-0-12-381300-8.00007-1. PMID: 21034975; PMCID: PMC7150010.
- Cesta, M. F. (2006). Normal Structure, Function, and Histology of Mucosa-Associated Lymphoid Tissue. Toxicologic Pathology, 34(5), 599–608. doi:10.1080/01926230600865531
- Cesta MF. Normal structure, function, and histology of mucosa-associated lymphoid tissue. Toxicol Pathol. 2006;34(5):599-608. doi: 10.1080/01926230600865531. PMID: 17067945.
- Mak, T. W., & Saunders, M. E. (2006). Cells and Tissues of the Immune Response. The Immune Response, 35–67. doi:10.1016/b978-012088451-3.50005-3
- Brandtzaeg, P., Kiyono, H., Pabst, R. et al. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol 1, 31–37 (2008). https://doi.org/10.1038/mi.2007.9
- Cesta, M. F. (2006). Normal Structure, Function, and Histology of Mucosa-Associated Lymphoid Tissue. Toxicologic Pathology, 34(5), 599–608. doi:10.1080/01926230600865531
- Pak, Kevin MD1; Junga, Zachary MD2; Davis, Joshua MD2; Young, Patrick MD; FACG, 2. S1614 Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma Isolated to the Colon. The American Journal of Gastroenterology: October 2020 – Volume 115 – Issue – p S828 doi: 10.14309/01.ajg.0000708504.66374.12
- Kuper, Christine & Wijnands, Marcel & Zander, Serge. (2017). Mucosa-Associated Lymphoid Tissues. 10.1007/978-3-319-47385-7_4.
- Niino, D., & Ohshima, K. (2012). Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma. In (Ed.), Hodgkin’s Lymphoma. IntechOpen. https://doi.org/10.5772/19557
- https://www.alliedacademies.org/abstract/primary-mucosaassociated-lymphoid-tissue-malt-lymphoma-of-the-minorrnsalivary-glands-in-the-upper-and-lower-lips-of-a-male-paediat-6871.html
- https://radiopaedia.org/articles/malt-lymphoma
- https://www.leukaemia.org.au/blood-cancer-information/types-of-blood-cancer/lymphoma/non-hodgkin-lymphoma/malt/
- https://empendium.com/mcmtextbook-sae/chapter/B78.II.4.9.?rfmcm
- https://amjcaserep.com/abstract/index/idArt/902843
- https://microbenotes.com/mucosa-associated-lymphoid-tissues-malt/#references
- https://www.histology.leeds.ac.uk/lymphoid/MALT.php
- https://emedicine.medscape.com/article/207891-overview


