Table of Contents
The most commonly used equipment is inoculation needles, transfer loops, inoculation, Bunsen burner, autoclave (or pressure cooker) incubators, hot air oven centrifuge, spectrophotometer magnetic stirrer electric shaker and rotary shaker heating plate, heating mantle distillation plant, UV-lamp carbon dioxide cylinder, water-bath and a single-pan balance that has weights (for general use) chemical balance, fine analytical balance pH meters, Quebec colony counter, Laminar air flow, camera lucida electrophoresis and a high-quality microscope and many more. To perform photomicrography, a photomicrographic camera mounted in a microscope equipped with every accessory is essential in the microbiology lab.
The other requirements: A large-sized container to store disposable items is essential in a laboratory for microbiology. Cotton rolls and forceps, scissors, blades, sealing films, cellotape, aluminum foil, parafilm, enamel tray, different kinds of containers including glass marker pen, brush, rubber, pencil measuring scale, Vernier’s Calliper, etc. are employed in laboratories for microbiology. A few of the tools and equipment are described in this document.
List of Instruments used in Microbiology Lab
- Inoculation needle and inoculation loop
- Bunsen burner (Spirit lamp)
- Waterbath
- Autoclave
- Laminar Air Flow
- Incubator
- Hot air oven
- Quebec colony counter
- The pH meter
- Balance
- Spectrophotometer (Colorimeter)
- Centrifuges
- Microscope
- Incinerator
- Deep Freezer (Laboratory Refrigerators and Freezers)
- Homogenizer
- Hot plate
- Magnetic Stirrer
- Vortex Mixture/ Vortexer
- Water Distiller
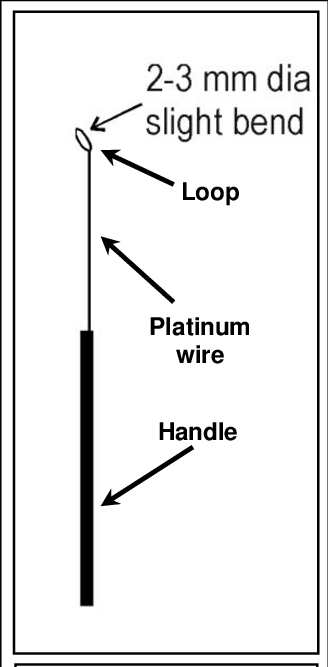
1. Inoculation needle and inoculation loop
- They are among the most frequently employed tools.
- The Inoculation needle/loop consists of a platinum wire that is welded to a metallic rod.
- Wire loops have an handle that is fitted with a steel screw shaft. Metallic rod nichrome, or platinum wire will be put into.
- The wire is be wrapped around a small circular object like a pencil or other similar objects. to create a loop making it twist mechanically. The loop must be designed as to keep an elongated film within it, by dips in the solution. In order to do this, a dimension (5-7 millimeters) that the wire has is suggested.
- Straight wire or straight needle is made of wire instead of loop. The free or open part of it is sharp. Straight and loop wire must be sterilized through either Bunsen burners or the hot heating coil until the loop or needle turn hot red. When the loop or wire has cooling, they are typically utilized for transferring cultures out of liquid broth.
- The straight needle is utilized to transfer the culture out of solid media. A smaller quantity of liquid culture may be controlled using a straight needle.
- The loop and wire can be are used to extract small amounts of solid material from a microbial colony and also to inoculate liquid or solid medium. Both the straight and loop wire need to be heated right after use, so that contamination is prevented.
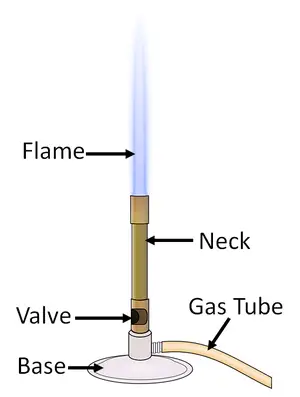
2. Bunsen burner (Spirit lamp)
- The primary device that provides warmth in laboratory experiments includes that of the Bunsen burner. It’s named in honor of its inventor, R. W. Burner. It is also referred to as a spirit lamp due to the use of spirits for burning purposes.
- The gas is introduced into the burner through the base and the supply is controlled externally through gas cock. As the gas is pushed upwards, it is able to enter at the bottom, it is drawn through those air intake ports located just below the top of the.
- The amount of air that is released can be controlled by turning an sleeve that is fitted over the openings in the body of the burning device.
- To stop the flame from escaping, certain tips are commonly placed over the top of the barrel.
- The best method for lighting your burner would be to shut off the supply of air, turn on the gas, and then light. The flame will appear large and yellow. Open the air intake slowly until the flame turns the blue color.
- For maximum heat , you can open it further to make two distinct zones emerge with the outer one is blue in color and the cone-shaped. The most hot point of the flame lies just above the middle zone.
- In certain instances, when the air flow for the combustion chamber is raised, the flame will blow out or separates itself from the burner’s tip. This indicates it is that gas flows are high and must be reduced. Before re-lighting, make sure to turn out the supply of air first. The inoculation loop is sterilized before being inserted into cultures tubes, or Petri plates.
- It is also utilized for the transfer and purification of microbial culture. When opening the cotton cap mouths of the tubes or flasks of culture should be heated to ensure the microorganisms , if they exist in the mouth are killed.
- The sterilization of tools using a the use of a spirit lamp is referred to as incineration.
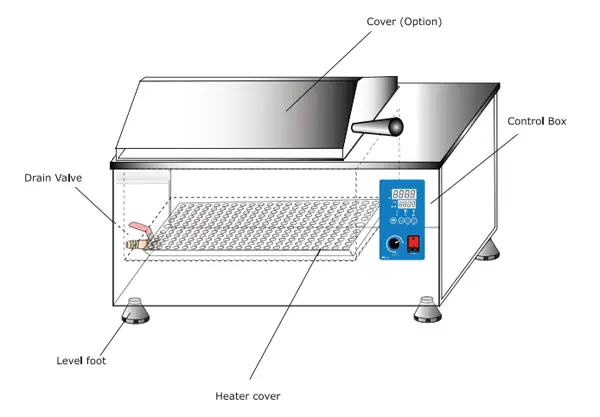
3. Waterbath
Read More: Working Principle of Laboratory Hot water bath with Operating Procedure.
- Waterbath is an instrument which is utilized to supply an unchanging temperature to the sample
- It’s a small insulating box made of steel, and fitted with an the electrode of an electric heating coil.
- It is also controlled via the thermostat.
- In some water-bath forms, the shape of the plate turns and is referred to as shakers in water-bath. It is more beneficial to microbiologists since it gives uniform heating to the sample materials intended to be used for incubation.
4. Autoclave
Read More: Autoclave Definition, Working Principle, Components, Operating procedure.
The killing effects of heat on living organisms can be achieved through the increase of steam pressure in an enclosed system. The water molecules are consolidated which results in an increase in their permeation. The water is boiling at 100 degrees and steam builds up in a closed vessel, leading to an increased pressure. This relationship between temperature and pressure is illustrated below.
- The autoclave is generally composed of pressure cookers composed of gun-metal sheets, which are held in the aluminum case (Fig. 1.10).
- It’s closed with a swing doors that are secured to the wall by bolts with a radical design.
- In laboratories for microbiology, a an autoclave with a horizontal system that is jacketed is essential.
- The steam flows from below at the bottom. The walls on the sides will be heated through the jacket. It is equipped to keep track of the pressure.
- There is a way to regulate the pressure with a the pressure meter. It is a security valves that protect against any accidents. It’s based on moist heat , which is can be used for sterilisation.
- The autoclave operates with fifteen Ib./inch^2 steam pressure, for 30 minutes. As shown in the table above. The temperature of 30 minutes will eliminate all spores and bacteria in microorganisms’ cells.
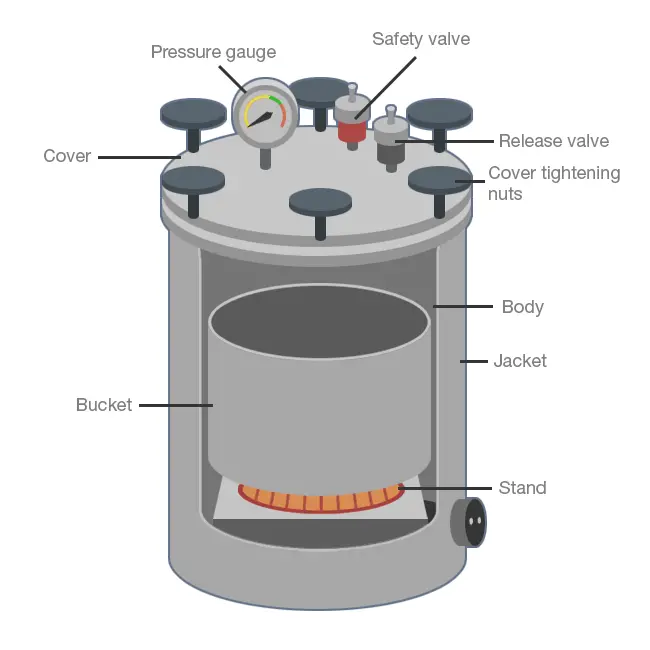
Precautions: The amount of water must be checked before starting the operation. The air must be completely evacuated and the steam should have access to the material to be sterilized. The glass or cotton beads are sterilised using an enclosed glass container that is sealed by foil. The substances that are heat sensitisers should not be sterilized by autoclaving. Today, checking the completeness of autoclaving is made possible using strips that have a red solution that turns green at the temperature of 115degC if it is left for 25 minutes. Bacilli spores after autoclaving, is considered as ideal if there is no growth found on thioglycollate medium the cooked medium for meat.
5. Laminar Air Flow
Read More: Laminar flow hood/cabinet Definition, Parts, Principle, Application
- Laminar flow is an instrument that is comprised of an air compressor located in the rear of the chamber, which is able to create air flow at a the same velocity across parallel lines of flow. It has a specific filtering system that is high-efficiency particles in the air (HEPA) that is able to remove particles that are as small as 0.3 millimeters.
- In front of the blower there is a mechanism which the air that is blown out of the blower creates air velocity along flow lines that are parallel.
- The concept of laminar flow relies on the flow of an air current that is uniform in velocity through parallel flow lines, which assist in the transfer of microbial culture under an aseptic condition. Air flows through filters and then into the enclosure. the filters do not permit any type of microbes to enter the system.
- Within the chamber, one fluorescent tube and another UV tube are installed. Two switches for these tubes as well as an additional switch to regulate of airflow are installed on the device. Because of the uniform speed and the parallel flow of air current the pouring of media, plating. slant preparations, streaking etc. Without any contamination are carried out.
- At first dust particles are removed off the surfaces of flow by using a smooth cloth that is infused with alcohol. Turn on the UV light for a time of 30 minutes in order to eliminate germs that are found in the work space.
- Front cover of device is open to keep the material you want to keep within. It is set to the appropriate level so that the air in the chamber is removed as the air in the chamber could be contaminated or carry contaminants.
- Place yourself comfortably in the middle of the chamber and wipe the table with alcohol in order to minimize the amount of dust. All work related to pouring or plating, streaking etc. must be completed within the flame area of the burner or lamp.
- In the microbiology laboratory, a the laminar airflow that is horizontal is utilized to deliver air through the filter.
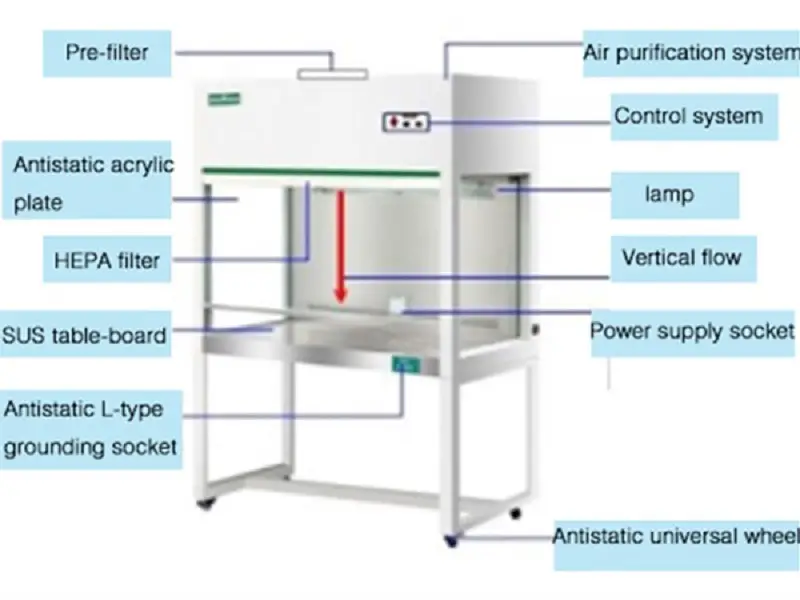
Precautions: Take off your shoes before you enter to operate the machine. Cleanse your hands using soap or detergent. You should not speak inside the chamber when performing Microbial culture transfer at all times, otherwise the chances of contamination could be higher and could come by sneezing, mouth, or through air.
6. Incubator
Read More: Incubator: Definition, Principle, Components, Types, Operating Procedure, Use.
- An incubator is a device comprised of a steel/copper chambers, in which air or warm water circulates by electrical current or through a small gas flames.
- Incubator temperature is maintained steady due to its control via a thermostat.
- The incubator is made of a double walled chamber that can be that can be adjusted to the desired temperature. This is accomplished by an external knob to control temperature control. The space between walls then insulated to test the heat conduction. A thermometer is placed from the top to record the temperature. Nowadays, sophisticated incubators are made available with oxygen and humidity control systems.
- The temperature has a significant impact on development of microbial. So, instruments are typically constructed to permit the microorganism that you wish to thrive at a specific temperature.
- It’s designed to allow the growth of microbial colonies in the appropriate medium at the right temperature. When using an incubator, difference in temperature shouldn’t exceed one degree.
- Smaller incubators with square shapes are more efficient than larger ones. If a temperature lower than that of the room is needed it is possible to circulate the water through the chamber before passing through an Ice chest.
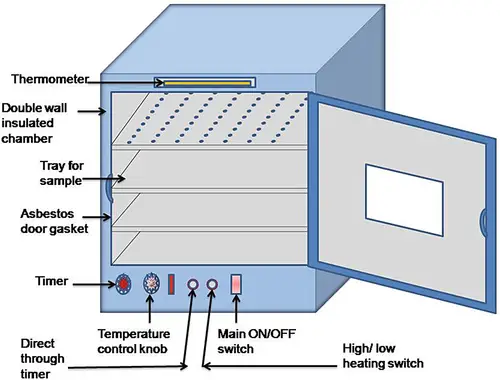
Precautions: The doors of the incubator must be shut only when it is necessary. In the event that the tube has set to be kept incubated for a prolonged period or at a higher temperature the medium could become dry due to the excessive transpiration. In these cases, a the plug of cotton should be placed into the neck of tube. The tube should be capped with a rubber cap that it covers the plug. When the Petri dishes are going to be kept for a lengthy period of period of time, they can be placed in a humid chamber with cotton wool that is sterile on the bottom.
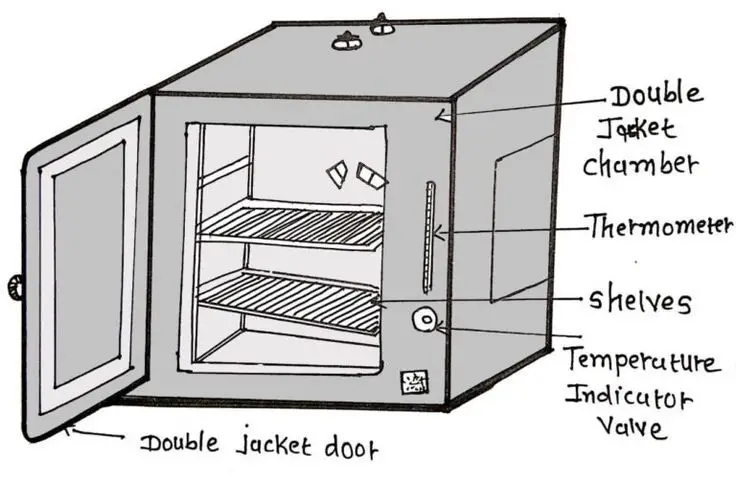
7. Hot air oven
Read More: Hot air oven Definition, Principle, Parts, Application, Procedure.
- The hot air oven is typically employed to sterilize glassware as well as other metal items that are damaged through autoclaving. In this case, dry heat sterilisation is utilized. Ovens operate on this idea. Although sterilisation can be achieved through autoclaving, glassware requires dry heat, not humid heat. The microbes are killed by burning its chemical component. It’s less effective contrasted to moist heat.
- Nowadays, ovens that are made of microwaves are offered in the market however, generally, they are not of the lab for long.
- The typical oven consists of double-walled chambers, where the space between two walls is shielded. It is heated below using electricity while heating elements are set up in a way to ensure uniform heating throughout the inside of the oven.
- The thermostat is built-in that is used when needed, and it assists in regulating temperature. The calibration knob is used to set the temperature that is desired.
- In order to sterilise, the hold duration is contingent on the temperature. If the temperature of the oven is 160 degrees Celsius The holding time must be one hour. At 180degC, it should be 30 minutes. The holding time could be less for better sterilisation.
- The glass material must be cleaned and dried before storing in the oven. Otherwise, it could break. When the time for holding has expired and the glassware shouldn’t be taken out at once, instead, the temperature must decrease so that the cool air of the outside is not able to break or damage the glassware.
- The air inside the oven, needs to be circulated through a fan to ensure that the oven’s components are maintained at the right temperature. The oven’s components must be placed in a way that they do not hinder the circulation of air.
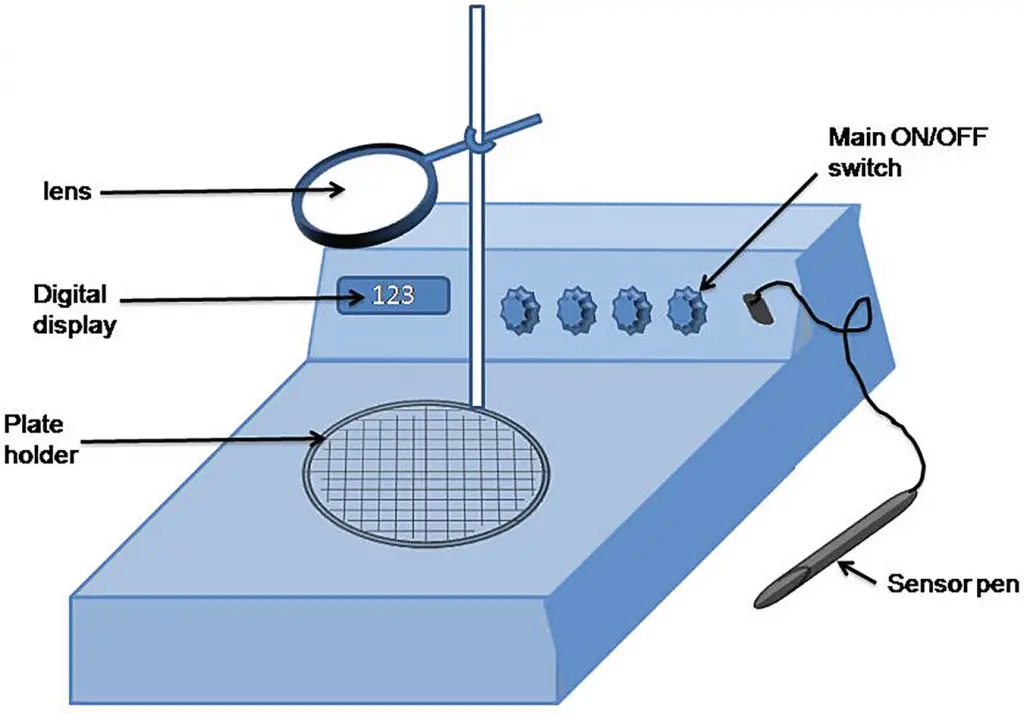
8. Quebec colony counter
- It is a method to measure the tiny or close-growing colonies that grow on the surface of the media.
- To ensure accuracy The lens that is installed or mounted within it will help to see the colonies.
- The lens can be moved on the box, and it can be adjusted to allow for the colonies to be seen.
- It is the Petri plate is positioned on a slanting platform designed to be used for it, and is illuminated using a the light source that is located beneath.
- The number of colonies is displayed using the aid of a digital reading meter.
9. The pH meter
- In the beginning, during 1909 Sorenson employed the word pH to refer to the concentration of hydrogen ions in the solution.
- pH can be described as the negatively logged hydrogen ions (H*) concentration: pH=-logo10 (H+) = 7
- pH is the amount of alkalinity and acidity in the solution, on a scale of from 1 to 14.
- pH values from 1 to 7 represent the acid values, pH 7 neutrality and pH 7-14 alkalinity. pH in water is 7, at 25 degrees Celsius.
- The acid is a proton donor while base is proton acceptor (acid = base + H+) i.e. acid dissociates and generates hydrogen ions (H+).
- Maintaining pH values is an essential factor in the growth process of all organisms.
- pH is measured using the pH meters, which are an instrument calibrated to several buffers of well-known pH values.
- A standard pH meters is equipped with two electrodes, one of which is a glass electrode, and the other being the secod mercury-mercurous chloride (calomel) or silver-silver chloride as a reference electrode. It is used as a reference electrode to placed within the saturated KCl solution.
- A typical pH gauge is comprised consisting of an electrode made from glass composed by a thin glass membrane that is selectively capable of permeation to H+
- The purpose of an electrodes is to provide a an electrically conductive bridge between the metallic element and the solution in which the two electrodes are positioned.
- Nowadays, pH meters with combined electrodes are also on the market.
- Utilizing the pH measuring device, pH of an unidentified solution can be measured.
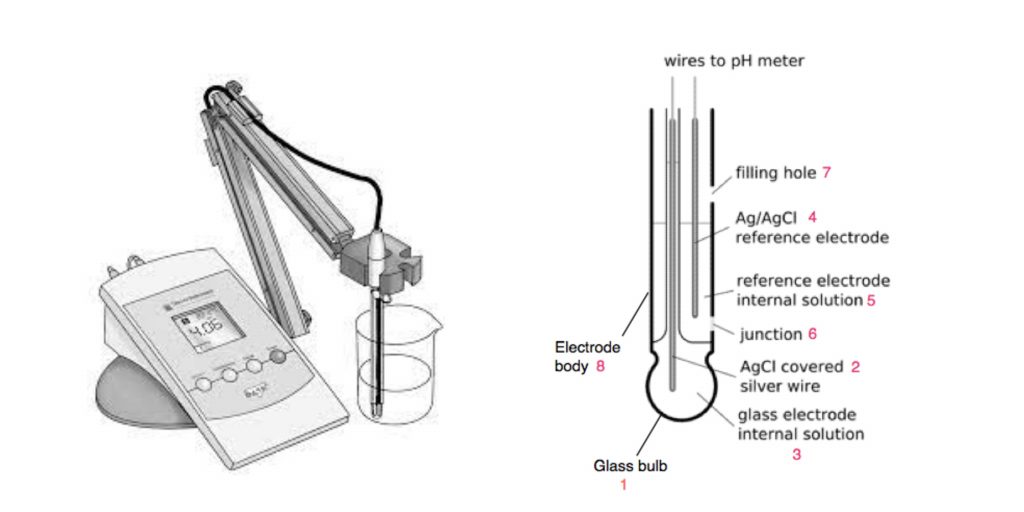
Precautions: A pH gauge must be handled with care so that the electrode will not be damaged. The electrodes should be kept in water that is distilled to ensure that the electrode will not be functional.
10. Balance
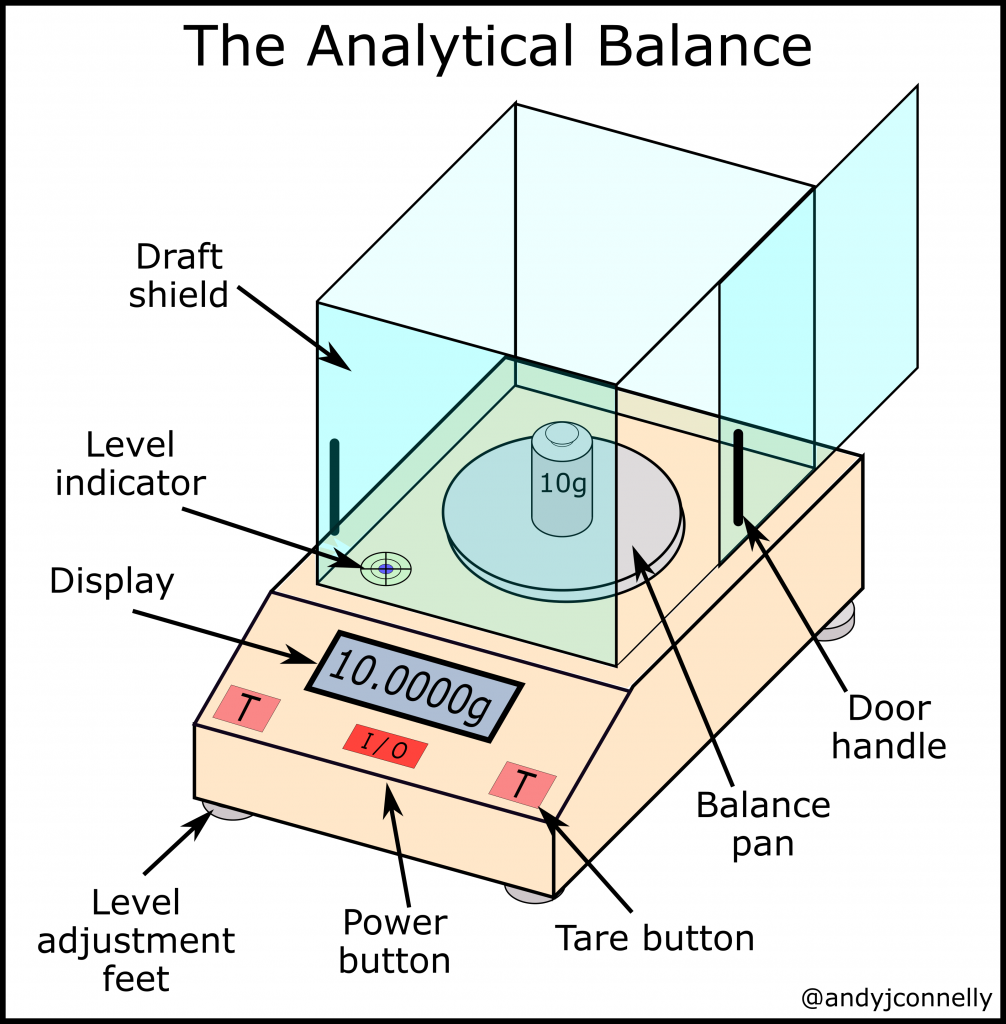
In experiments with biological or chemical substances the precise amount of chemical needs to be measured using an instrument. However, any experiment cannot be carried out without a balanced. There are various kinds of balances for weighing including single pans, chemical or analyticalbalances and electrical ones. These balances are applied in accordance with the amount of material to be measured. For instance, a single pan balance can be used to weigh an quantity of more than 100 grams A chemical or electrical balance can be utilized to weigh a 10 mg however, its total weight capacity is 100 grams. Additionally the ultramicro balance is able to weigh any materials from 0.01 up or 2 milligrams.
Electrical balance
- It works with the help of electricity and displays a the digital weights display.
- It is comprised of a single pan that weighs a single pan. Its weight is counterbalanced with weights, and is set to zero.
- The weighted material is put on the balance pan and the counterweights required are removed using the knobs. As time passes, the digital scale starts moving between up and down.
- Always take off the counter-weights that are proportional to the material’s weight.
11. Spectrophotometer (Colorimeter)
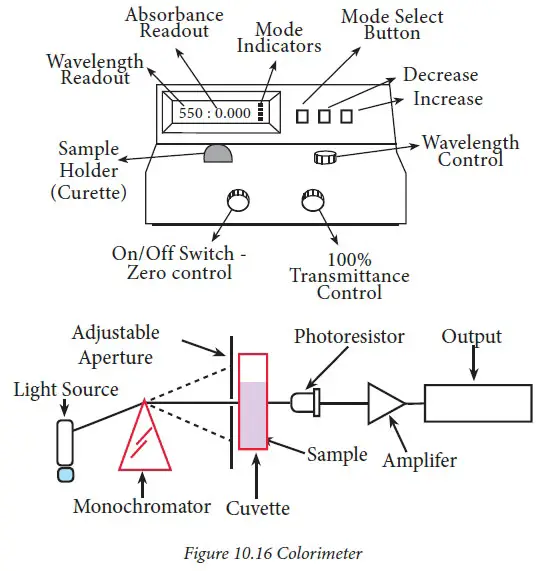
- Spectrophotometer is a tool which makes use of the light source as an instrument for radiation. It also measures changes in optical density , or absorbance.
- It is based on three fundamental principles: (i) the radiation source, (ii) a unit to disperse radiation across various wavelengths and (iii) an instrument that can will detect how much radiation is present that is detected at different wavelengths.
- Spectrophotomete employs the monochromatic (narrow frequencies) radiation, while colorimeter employs wide wavelengths.
- There are a variety of atoms as well as their electron clouds within every chromophore (a chemical molecule or a portion that is). Because of changes in the energy levels of electrons, the form of the chromophore can be altered. These variations in energy are recorded using any spectrophotometer.
- To quantify the absorption of various concentrations of a compound can be measured using Beer’s Law (absorbance is directly proportional to the concentration of the substance i.e. when a monochromatic light passes through an absorbent medium it’s intensity decreases exponentially when the concentration of the medium that absorbs it increases). The mathematical formulation of Beer’s law is: Log Lo/L = Kc. In which lo is the original intensity of beam of light I= intensity of the beam that passes through the solution, C = Concentration on the solution as moles/l K= Continuous A graph of absorption (Y-axis) against concentration (X-axis) provides the calibration curve. This illustrates the variation that Beer’s Law. It is a good tool for quantitative estimation.
- The spectrophotometer comes in many varieties, such as (i) the visible range spectrum spectrophotometer (contains the lamp (36 watts, 12 volts) to provide light. It makes use of all seven light spectrums that spans from violet to red (VIBGYOR) which ranges between 400 and 1,000 nm), (ii) UV visible spectrophotometer ( includes a tungsten bulb or deuterium lamp which emits UV light. It utilizes wavelengths of 200 to 1000 nm of light) as well as (iii) IR spectrophotometer (the filaments made of tungsten can be used as a the light source. It is based on 1000-3000 nanometers infra-red (IR) wavelength).
12. Centrifuges
The centrifuge is a device which rotates at a high rate and separates particles or substances by the density and mass with the help by centrifugal force. The force exerted by the centrifuge is measured in terms of revolutions every minutes (rpm) of the angular speed. A centrifuge is comprised of the “head” which is rapidly rotated with an up-right motor. Usually, four containers or cups are connected to the head to hold the tubes and other vessels made of the material that the particulate matter will be separated. When centrifugation is performed, the liquid that contains particles is kept inside tubes and moves at a specific speed, and, when the centrifugation process is completed the particles are settled on lower levels of tubes. The various types of centrifuges typically used include slow-speed (clinical centrifuge) and high-speed, also known as ultracentrifuge and superspeed. The maximum speed limits of ultracentrifuges with low-speed, high-speed or low-speed speeds are 5500 rpm, 18000 rpm and 20000-60000 rpm and 20000 to 60000 rpm, respectively. They are utilized for the separation of particles in suspended matter removal of liquid mixtures with varying in density and solids or liquids the concentration of microorganisms within various samples for studies of enzymatic activity.
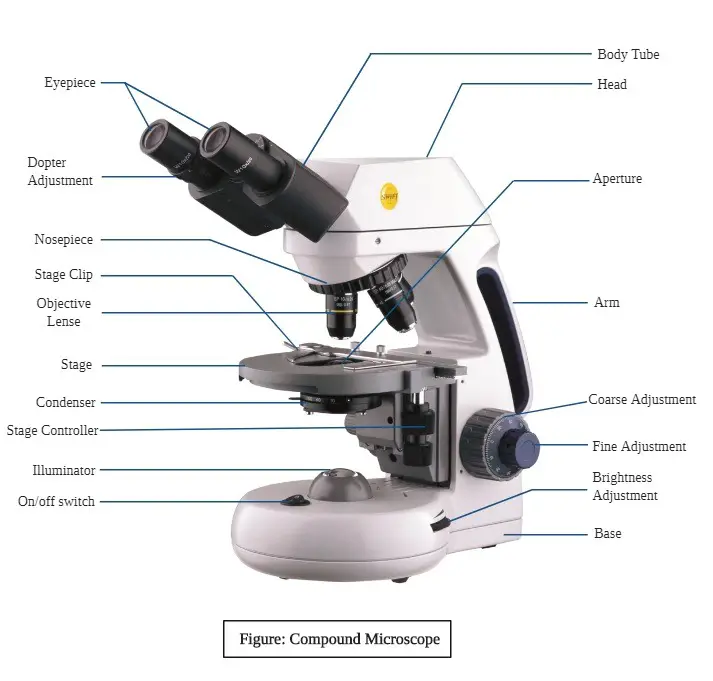
13. Microscope
For the study of basic and applied microbiology. The microscope is an essential tool in all the tools needed in the laboratory. The microorganisms in tiny sizes are visible only through a microscope and are inaccessible to naked eye. Some of them are colorless, but the microscope with a the phase contrast attachment can see them with high resolution. Antoni van Leeuwenhoek (1673) invented in the field of science biconcave lenses that were positioned between two plates made of steel that had a magnification of 300X as a microscope.
The microscope was utilized by him to observe microbes. The electron microscope today is capable of magnifying over 250,000X of the specimen. There are two main kinds of microscopes are offered that include light microscopes, which comprise dark field, bright field as well as phase contrast and electron microscopes and fluorescent microscopes, its function is based on the electron beam and magnets to view submicroscopic images. The most basic microscope is comprised of an eyes (10X magnification) which is inserted inside the tube of the microscope.
The magnification is different in the the ocular eyepiece. For identification, additional lower power lens called objective are utilized, which have various power (10X 40X, 10X). To increase magnification, an oil-immersion (100X) may also be utilized. These are necessary to study of microbes at high magnification. An oil immersion lens can bring the objective closer to focus when it is rotated by the nosepiece carrier. The fine details are readily discernible with the an oil infusion (100X) goalie. The other component of the microscope is stage, which has the use of a mechanical device to hold it or clips. In certain microscopes the mechanical stage could be changed and the content in the Petri dish can be examined using lower power.
It is the “Abbe type” condenser is essential when using oil immersion objective lenses because it provides the necessary light. It features an iris diaphragm as well as an optical filter that can be moved below the lens of the objective. Condenser is made up of aplanatic lensesand have an aperture that ranges from 1.3 to 1.4 when using high resolution. The microscope features the light source (lamp) which is either within or externally separately moveable device underneath the condenser.
This device, when set in a fixed position, it is able to direct light upwards by tilting. Utilizing a lamp with a lens creates a clear images, which improves studying the microorganism’s details. Two types of preparations, wet mount and stained smear placed on the slide’s surface with glass covers, are employed for microscopy that is simple. Wet mount preparations are employed with objectives (10X 40X) and for more detailed observation, an oil immersion objectives are suggested.
A stained smear is used to locate the specimen with dry objectives (10X, 40X), whereas the smear covered with immersion oil provides a clear and translucent image of the bacteria/microorganism. You can also see the images of the bacteria directly by focusing on oil however it requires technical expertise and sharp power of observation. The steps to operate the basic microscope are described below.
- Set the smear onto the stage, then concentrate on the image.
- To study a specimen with the aid of an eyepiece or objective lens. During the initial stage , make sure to keep the condenser to the level of the stage in the scope.
- Open the aperture diaphragm completely of the condenser. Then, by tilting the mirror focus , you can move the optical Axis.
- Examine the picture of the specimen in sharp focus using the fine control of the lens. The majority of the time, a flat mirrors are employed and for lower power concave mirrors are used. Change the angle of the condenser to increase the light and aperture diaphragm to obtain a clear as well as the best contrast from the smear.
- Determine the exact position of the condenser most suitable to focus the smear. Take off the evepiece and looking through the tube to the visible light.
- Make sure you get a good light after adjusting the condenser’s location. Furthermore tilting the mirror will aid in the illumination.
- Reduce the scattered light that comes from the lenses by closing aperture diaphragm on the condenser. It is easy to change the nosepiece on to another objective lens and then look at the the slide’s surface with the eyepiece. Then, focus it on the final adjustment to get the most optimal light. It is worth noting that the focal point of one objective is almost the same as that of other when the lenses are of a single manufacturer. A mismatch in the focal position is , therefore. possible. It is therefore necessary to lift the tube slightly prior to focusing the high-power (40X) (or oil immersion (100X) into the proper position.
- Be careful when using lenses for focus above the objective which are adjustable using a side view, then lower the objective slowly on oil drop and then down just a bit in that it will be able to touch the drop of oil onto the slide. If the specimen is found using fine adjustment, search for the specimen, then raise the objective by with the large adjustment wheel. It is recommended that prior to placing the slide onto the platform,, apply an oil drop onto the smear. It is also helpful to apply the drop of oil onto the slide using the nosepiece rotated half in the direction of the desired objective before moving the oil-immersion goal into place.
- Generally speaking, focusing an image while in oil requires some practice. If the specimen is situated in a specific area, you can use a fine adjustment wheel to get a sharp and clear image of the specimen. There is a good chance of breaking the slide while focusing in oil immersion. This is why extreme diligence is needed. The dull, dim and blurred image indicates something is not right in the lens. This is why it is important to clear the objective by using lens paper. Sometimes, it’s necessary to examine the tubes and eyepieces too. One of the most common causes that causes haziness is an air bubbles that are trapped in the oil of immersion. There is a way to eliminate these bubbles first by raising your objective and then turning the eyepiece. clean the oil off with lens paper , then turning the eyepiece back and focus again. Sometimes, closing the diaphragm may also cause an image that is not as sharp.
14. Incinerator
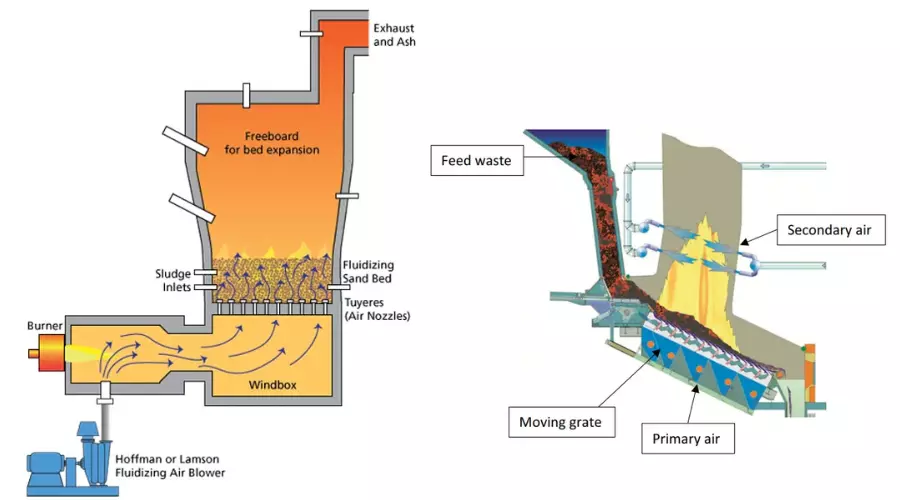
A laboratory incinerator is a specialized type of incinerator specifically designed for the disposal of laboratory waste. Laboratory waste can include various types of hazardous materials, such as chemicals, contaminated materials, biological waste, and other substances that require proper handling and disposal.
The purpose of a laboratory incinerator is to safely and effectively destroy laboratory waste, preventing its release into the environment and reducing the risk of contamination or exposure. These incinerators are typically smaller in size compared to industrial-scale incinerators and are designed to meet the specific needs and regulations of laboratory settings.
Here are some key features and considerations of a laboratory incinerator:
- Size and Capacity: Laboratory incinerators are available in different sizes to accommodate the waste generation of specific laboratories. They can range from small benchtop units suitable for individual workstations to larger units capable of handling higher volumes of waste.
- Temperature Control: Laboratory incinerators allow for precise temperature control to ensure efficient and complete combustion of laboratory waste. The temperatures employed are typically high enough to effectively destroy hazardous substances.
- Air Pollution Control: Laboratory incinerators are equipped with air pollution control systems to minimize the release of harmful emissions. These systems may include filters, scrubbers, or catalytic converters to remove pollutants and ensure compliance with environmental regulations.
- Safety Features: Laboratory incinerators incorporate safety features to protect laboratory personnel and prevent accidents. These features may include temperature monitoring, automatic shut-off systems, and safety interlocks to ensure safe operation.
- Waste Types: Laboratory incinerators can handle a variety of waste types commonly generated in laboratory settings, including chemical waste, contaminated materials, biological waste, and disposable lab equipment. However, it’s important to follow local regulations and guidelines for waste segregation and disposal.
- Compliance and Regulation: Laboratory incinerators must adhere to specific regulations and guidelines regarding waste disposal, emissions control, and worker safety. Compliance with local, state, and national regulations is essential to ensure responsible waste management practices.
It’s crucial for laboratory personnel and management to be knowledgeable about the proper use and maintenance of laboratory incinerators. Training on waste segregation, incinerator operation, and safety protocols should be provided to ensure effective and safe utilization of these systems.
Note: It’s worth mentioning that the use of laboratory incinerators may vary depending on the specific regulations and practices in different regions. It’s essential to consult local authorities and comply with applicable regulations for the proper disposal of laboratory waste.
15. Deep Freezer (Laboratory Refrigerators and Freezers)
A laboratory deep freezer, also known as an ultra-low temperature (ULT) freezer or cryogenic freezer, is a specialized piece of equipment used to store and preserve sensitive biological samples, chemicals, and other temperature-sensitive materials at extremely low temperatures. These freezers are commonly found in research laboratories, pharmaceutical companies, and biomedical facilities.

Here are some key features and characteristics of a laboratory deep freezer:
- Temperature Range: Laboratory deep freezers are capable of reaching and maintaining ultra-low temperatures typically ranging from -80 degrees Celsius (-112 degrees Fahrenheit) to as low as -150 degrees Celsius (-238 degrees Fahrenheit). The precise temperature control is crucial for long-term preservation of delicate samples.
- Sample Storage: These freezers are designed to provide a controlled environment for the storage of various samples, including biological materials, enzymes, DNA/RNA samples, vaccines, pharmaceuticals, and other temperature-sensitive substances. They often have multiple compartments or shelves to accommodate different sample sizes and types.
- Insulation and Cooling System: Laboratory deep freezers are constructed with high-quality insulation materials to minimize temperature fluctuations and prevent heat transfer. They are equipped with efficient cooling systems, typically using compressors and refrigerants, to maintain the desired low temperature consistently.
- Alarm and Monitoring Systems: To ensure the safety and integrity of stored samples, laboratory deep freezers are equipped with alarm systems. These alarms can detect temperature deviations, power failures, or other malfunctions and alert users to take immediate action. Some freezers also have monitoring features, such as digital displays or data logging capabilities, to track temperature history.
- Security and Access Control: Deep freezers may include security features such as locks or access control systems to restrict unauthorized access and protect valuable or sensitive samples from theft or tampering.
- Backup Systems: Given the critical nature of the samples stored in laboratory deep freezers, some models may have backup systems such as backup power supplies or redundant cooling systems to ensure sample integrity in case of power outages or equipment failures.
It’s important to note that proper maintenance and regular monitoring of laboratory deep freezers are essential to ensure their reliable performance. Routine maintenance includes defrosting, cleaning, and periodic calibration of temperature control systems.
Additionally, it’s crucial to follow good laboratory practices when using deep freezers, such as labeling samples correctly, using appropriate containers, and maintaining an organized inventory system. This helps prevent cross-contamination, sample loss, and retrieval difficulties.
When selecting a laboratory deep freezer, it’s essential to consider factors such as the required temperature range, capacity, energy efficiency, alarm systems, and any specific requirements for your particular samples or research needs.
16. Homogenizer
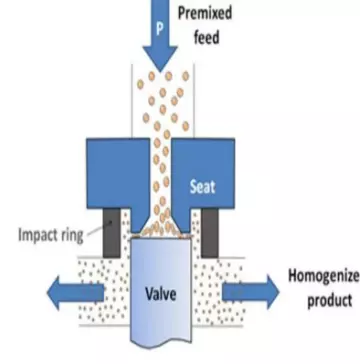
A homogenizer is a laboratory or industrial equipment used to break down and mix substances into a uniform and consistent mixture. It is commonly used in various scientific, research, and industrial applications, particularly in fields such as biology, chemistry, food processing, and pharmaceutical manufacturing.
The primary purpose of a homogenizer is to disrupt or reduce the size of particles, droplets, or solids in a sample and disperse them evenly throughout a liquid or semi-solid medium. This process helps achieve uniformity, improve product quality, enhance stability, and facilitate further analysis or processing.
Here are some key features and components of a homogenizer:
- Motor/Base Unit: The motor/base unit provides the power to drive the homogenization process. It typically consists of an electric motor that generates rotational or reciprocating motion and a control panel for adjusting speed, time, and other operating parameters.
- Homogenizing Tool/Probe: The homogenizing tool, also known as a probe or generator, is the part of the homogenizer that directly interacts with the sample. It can be a rotor-stator assembly, a high-speed blade, or a sonication probe, depending on the specific type of homogenizer. The design of the tool determines the mechanism used for homogenization.
- Sample Container/Vessel: The sample container or vessel holds the sample that needs to be homogenized. It can vary in size and material depending on the application and volume of the sample. The container should be compatible with the homogenizer’s tool and capable of withstanding the mechanical or sonic forces applied during the homogenization process.
- Homogenization Mechanism: Homogenizers utilize different mechanisms to achieve sample homogenization. Some common types include:
- Mechanical Homogenizers: These use mechanical forces to disrupt particles and create a uniform mixture. Examples include rotor-stator homogenizers, bead mills, and high-pressure homogenizers.
- Ultrasonic Homogenizers: These use high-frequency sound waves to create intense vibrations, leading to cavitation and the disruption of particles. Ultrasonic homogenizers are also known as sonicators.
- Pressure Homogenizers: These apply high-pressure forces to the sample, passing it through narrow channels or valves to generate shear forces and break down particles. High-pressure homogenizers are an example of this type.
- Cooling Systems (Optional): In some high-intensity homogenization processes, a cooling system may be included to prevent sample heating during the operation. This is particularly important for temperature-sensitive samples.
- Safety Features: Homogenizers may include safety features such as automatic shut-off mechanisms, overheat protection, or safeguards to prevent injury from moving parts or high-pressure systems.
Homogenizers are versatile tools and can be used for various applications such as cell disruption, emulsification, particle size reduction, dispersion, and extraction. The selection of a homogenizer depends on factors such as the nature of the sample, required degree of homogenization, sample volume, and desired throughput.
It is important to follow the manufacturer’s instructions and recommended protocols when using a homogenizer to ensure safe operation and achieve the desired homogenization results.
17. Hot plate
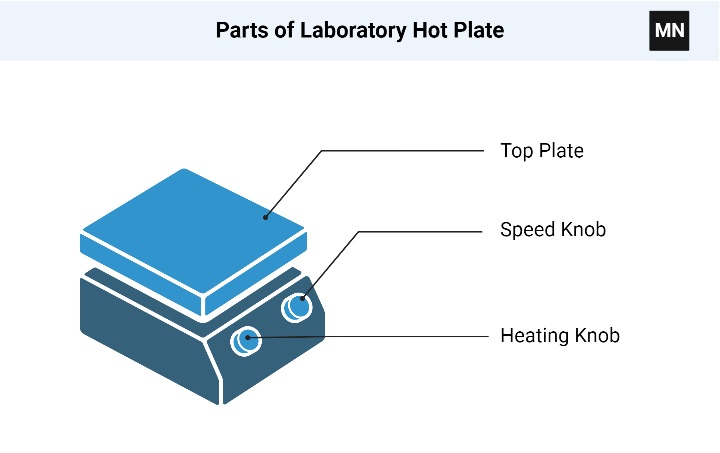
A laboratory hot plate is a commonly used piece of equipment in scientific research, educational laboratories, and industrial settings. It provides a flat, heated surface for heating and maintaining the temperature of substances in laboratory vessels such as beakers, flasks, or test tubes. Hot plates are widely used for various applications, including heating, boiling, evaporation, and gentle mixing of solutions.
Here are some key features and components of a laboratory hot plate:
- Heating Surface: The heating surface of a hot plate is typically made of a durable and heat-resistant material, such as ceramic, aluminum, or stainless steel. The surface is flat to provide uniform heating and is designed to withstand high temperatures.
- Heating Element: The heating element is located beneath the heating surface and generates heat to raise the temperature of the surface. The most common heating elements used in laboratory hot plates are electrical resistance coils or ceramic heating elements.
- Temperature Control: Hot plates feature temperature control settings to adjust and maintain the desired temperature. They usually have a control knob, digital display, or touchpad for setting and monitoring the temperature accurately. Some advanced models may include temperature feedback and automatic temperature regulation for precise control.
- Stirring Capability (Optional): Some hot plates come with integrated magnetic stirrers. These have a built-in magnetic stir bar that can be placed in the liquid being heated. The hot plate creates a rotating magnetic field, causing the stir bar to spin and provide gentle mixing or stirring of the solution.
- Safety Features: Hot plates often incorporate safety features to prevent accidents and ensure user safety. These may include:
- Temperature Limiting: A maximum temperature limit can be set to prevent the hot plate from exceeding a certain temperature, reducing the risk of overheating or damage to the sample.
- Overheating Protection: Hot plates may have built-in mechanisms that automatically shut off the heating element if the temperature exceeds a safe limit.
- Hot Surface Warning: Some hot plates have indicators or warning lights to alert users when the heating surface is hot, even if it is not actively heating.
- Stability and Non-Slip Features: Hot plates are designed with stable bases and non-slip feet to ensure that the unit remains steady during use.
- Power Control: Hot plates are electrically powered and typically connect to a power source using a power cord. They may have adjustable power control to regulate the heating intensity or a power switch for convenient on/off operation.
When using a laboratory hot plate, it’s important to follow good laboratory practices, handle hot objects with caution, and use appropriate protective equipment, such as heat-resistant gloves or safety goggles. Additionally, it is crucial to be aware of the flammability and compatibility of the materials being heated to avoid hazardous situations.
Overall, laboratory hot plates provide a reliable and efficient means of heating and maintaining the temperature of various substances in laboratory settings, facilitating a wide range of experimental procedures and processes.
18. Magnetic Stirrer
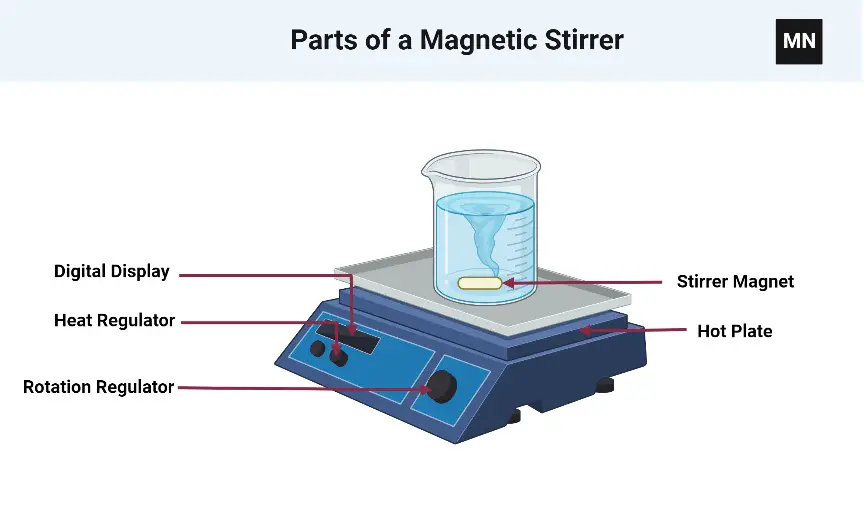
A magnetic stirrer is a laboratory device used to stir or mix solutions using a rotating magnetic field. It consists of a flat or cylindrical plate with a magnetic element embedded beneath it, and a stir bar, typically made of Teflon-coated magnetic material, which is placed in the liquid to be stirred. When the magnetic stirrer is turned on, the rotating magnetic field causes the stir bar to spin, creating a vortex and effectively stirring the solution.
Here are some key features and components of a magnetic stirrer:
- Stirring Platform: The stirring platform is usually made of a chemically resistant material, such as glass, ceramic, or aluminum. It provides a flat or contoured surface where the liquid-containing vessel can be placed for stirring.
- Magnetic Element: The magnetic element is located beneath the stirring platform. It typically consists of a rotating magnet or a set of rotating magnets driven by an electric motor. The rotating magnetic field produced by the magnets is responsible for the rotation of the stir bar.
- Stir Bar: The stir bar, also known as a flea or magnetic flea, is a small bar-shaped magnet coated with a chemically inert material, such as Teflon. It is placed in the liquid to be stirred and magnetically coupled with the rotating magnetic field from the magnetic element. The shape and size of the stir bar can vary depending on the volume and nature of the solution.
- Speed Control: Magnetic stirrers feature speed control settings to adjust the rotation speed of the stir bar. The speed control can be adjusted using a control knob, dial, or digital display, allowing users to select the desired stirring intensity.
- Heating Capability (Optional): Some magnetic stirrers come with integrated heating elements, allowing simultaneous stirring and heating of the solution. These units are known as hotplate magnetic stirrers or heating magnetic stirrers. The heating element is typically located beneath the stirring platform and can be controlled independently from the stirring function.
- Safety Features: Magnetic stirrers may include safety features to ensure user safety and prevent accidents. These features can include:
- Overheating Protection: If the temperature of the heating magnetic stirrer exceeds a certain limit, it automatically shuts off or reduces the heating power to prevent overheating.
- Stir Bar Detection: Some magnetic stirrers have sensors that detect whether a stir bar is present on the platform. If no stir bar is detected, the device may display a warning or prevent the heating or stirring function from operating.
- Non-Slip Surface: The stirring platform may have a non-slip surface or rubberized coating to keep the vessels stable during stirring.
Magnetic stirrers are commonly used in various scientific and research applications, such as chemical synthesis, sample preparation, and general laboratory mixing. They provide efficient and consistent mixing without the need for direct mechanical contact with the solution, minimizing the risk of contamination. It’s important to choose a magnetic stirrer that suits the specific requirements of the experiment, considering factors such as stirring capacity, speed range, heating capabilities, and compatibility with the vessel and solution being used.
19. Vortex Mixture/ Vortexer
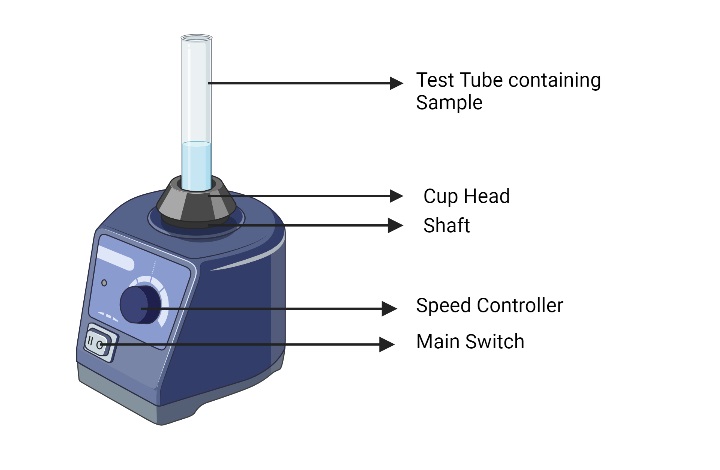
A vortex mixer, also known as a vortexer or vortex shaker, is a common laboratory device used to mix or vortex small volumes of liquid in tubes or vials. It creates a swirling or vortex motion in the sample by rapidly shaking or vibrating the platform on which the tubes or vials are placed. Vortex mixers are commonly used in biological, chemical, and clinical laboratories for tasks such as sample mixing, reagent resuspension, and cell culture.
Here are some key features and components of a vortex mixer:
- Mixing Platform: The mixing platform of a vortex mixer is a flat or cupped surface where the tubes or vials are placed. It is usually made of chemically resistant materials such as rubber or silicon to provide a stable and non-slip surface for the containers.
- Vortexing Motion: The vortexing motion is created by the rapid shaking or vibrating action of the mixing platform. This motion causes the liquid in the tubes or vials to spin around in a vortex, enabling thorough mixing or resuspension of the sample.
- Control Settings: Vortex mixers typically have control settings to adjust the intensity and duration of the vortexing motion. These settings can be adjusted using a control knob, switch, or digital display, allowing users to customize the mixing conditions according to their specific requirements.
- Adapters and Inserts: Vortex mixers often come with various adapters or inserts to accommodate different types and sizes of tubes or vials. These adapters help secure the containers in place during vortexing, ensuring proper mixing and minimizing the risk of spillage.
- Hands-Free Operation (Optional): Some vortex mixers feature a hands-free operation mode where the mixing platform can be activated by pressing the sample container against a pressure-activated switch. This allows for convenient and effortless mixing without the need to hold down the containers manually.
- Safety Features: Vortex mixers may incorporate safety features to protect the user and the equipment. These can include:
- Speed Control Lock: Some vortex mixers have a speed control lock to prevent unintentional adjustments or accidental changes during operation.
- Overload Protection: If the motor of the vortex mixer becomes overloaded due to excessive resistance or improper usage, it may have a built-in mechanism to automatically shut off or reduce the speed to prevent damage.
Vortex mixers are suitable for mixing a wide range of liquid samples, including reagents, buffers, solvents, cell suspensions, and enzyme solutions. They are particularly useful when working with small volumes of samples in tubes or vials, as they provide quick and efficient mixing without the need for extensive manual shaking.
When using a vortex mixer, it’s important to ensure that the tubes or vials are securely positioned on the mixing platform to prevent spills or accidents. Additionally, it’s advisable to use appropriate tube caps or closures to minimize the risk of leakage or sample contamination.
Overall, vortex mixers are versatile and reliable tools commonly found in laboratory settings, offering efficient and convenient sample mixing for a variety of applications.
20. Water Distiller
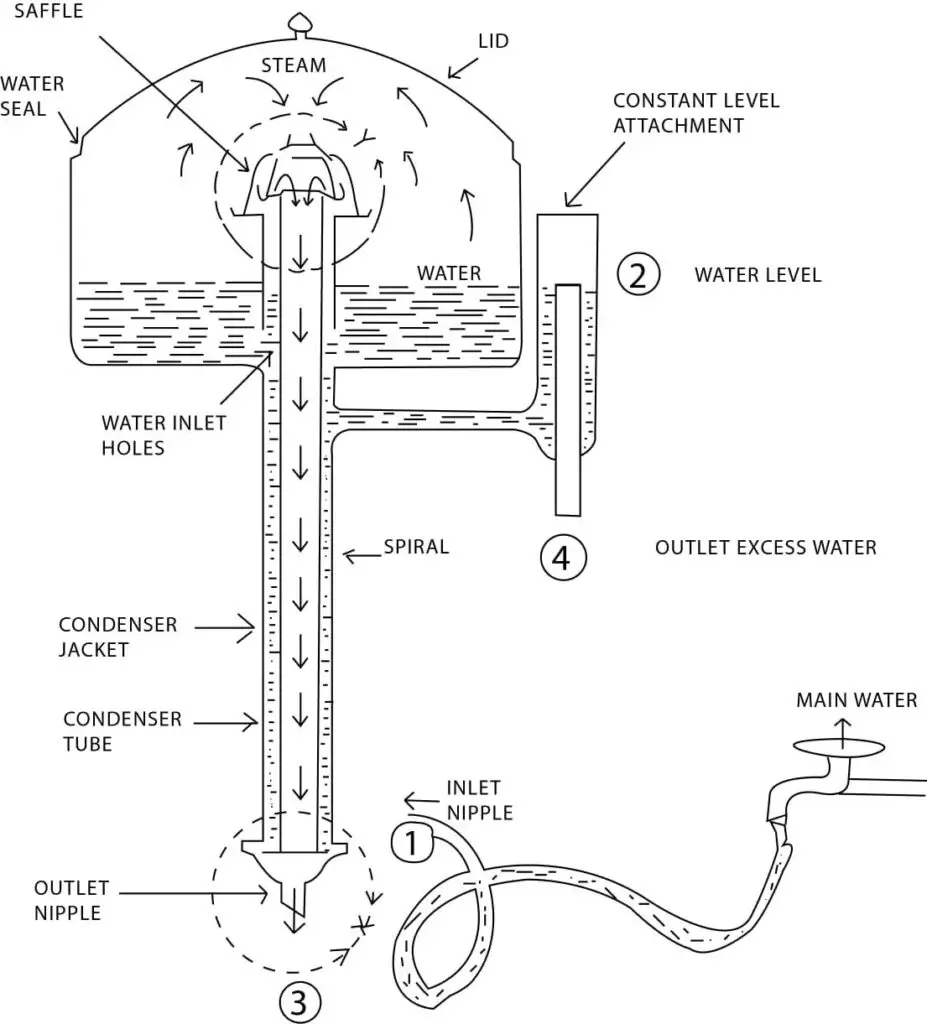
A water distiller is a laboratory or household device used to purify water by removing impurities, contaminants, and minerals through the process of distillation. Distillation is a method of separating components based on their boiling points. Water distillers heat water to its boiling point, collect the resulting steam, and then condense it back into liquid form, leaving behind impurities and contaminants.
Here are some key features and components of a water distiller:
- Boiling Chamber: The boiling chamber is where water is heated to its boiling point. It is typically made of stainless steel or heat-resistant glass to withstand high temperatures.
- Heating Element: The heating element, usually an electric coil, heats the water in the boiling chamber, bringing it to its boiling point and initiating the distillation process.
- Condenser: The condenser is responsible for converting the steam produced during boiling back into liquid form. It is a cooling component that allows the steam to lose heat and condense into purified water. The condenser may consist of coils or tubes through which cool water flows.
- Collection Container: The purified water collected after condensation is typically stored in a separate container or reservoir. This container is usually made of food-grade materials such as glass or plastic and may have a spout or valve for convenient dispensing.
- Filtration System (Optional): Some water distillers include an additional filtration system to further purify the water. This can include activated carbon filters, post-distillation filters, or UV lamps to remove any residual impurities and enhance the taste and quality of the water.
- Control Panel: Water distillers may have a control panel with various settings and indicators. These settings can include power controls, temperature controls, and timers to facilitate the distillation process and provide user control.
Water distillers offer several advantages in water purification:
- Effective Removal of Contaminants: Distillation is a reliable method for removing a wide range of impurities, including minerals, heavy metals, organic compounds, bacteria, viruses, and dissolved solids.
- Production of Pure Water: The distillation process produces highly purified water that meets or exceeds the standards set by regulatory bodies.
- Versatility: Water distillers can be used to purify various water sources, including tap water, well water, or brackish water.
- Independence from External Water Sources: Water distillers do not rely on a continuous supply of water and can produce purified water as long as they have an adequate water source and power supply.
It’s important to note that while water distillers are effective at removing most impurities, they may not eliminate volatile organic compounds (VOCs) with lower boiling points than water. Additionally, the distillation process removes beneficial minerals along with the impurities, so it’s advisable to consider remineralizing the water before consumption if necessary.
Water distillers require regular maintenance, such as descaling the boiling chamber and replacing filters if applicable, to ensure optimal performance and prolong the lifespan of the device. It’s essential to follow the manufacturer’s instructions and recommended maintenance schedule.
Overall, water distillers provide a reliable and convenient method for obtaining purified water, making them useful in laboratories, households, and other settings where clean and contaminant-free water is required.
